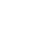This Fact Sheet has been organized into the following subsections:
- Section 1: Introduction
- Section 2: Individual Chemical Compound Analysis
- Section 3: Analytical Methods for Chemical Classes
- Section 4: Methods for Biological Contaminants
- Section 5: Analysis of Particulates
- Case Study: Effect-Directed Nontarget Analysis Identifies 6PPD-q as Cause of Urban Runoff Mortality Syndrome
1. Introduction
Reliable analysis of a contaminant in environmental media requires a high degree of confidence in its identity and its quantitation. This is normally achieved using reference materials such as analytical standards for chemicals and genomic sequence libraries for pathogens. In lieu of such reference materials, as is often the case for contaminants of emerging concern (CEC), identification and, subsequently, quantitation in environmental samples are uncertain. CEC are typically compounds or substances that one is previously unaware of (i.e., either in identity or in effect), but may (known-unknown) or may not (unknown-unknown) understand through similar compounds or substances (Figure 1). For example, true unknown-unknowns in the environment can include substances such as a transformation product of a known synthetic chemical (Tian et al. 2020) or a new strain of an existing pathogen, both of which are unlikely to have reference materials for identity confirmation and quantitation.

Figure 1. Knowns and unknowns. The matrix of knowns and unknowns (a.k.a. the “Rumsfeld Matrix”) can be used to map uncertainty in the identification and quantitation of CEC. CEC are typically compounds or substances that one is previously unaware of but may (known-unknown) or may not (unknown-unknown) understand through similar compounds or substances. The analysis of a CEC, when first encountered, follows a process of elimination whereby a full suite of targeted methods is exhausted to rule out known-knowns. CEC analysis then falls into the right two quadrants of the matrix where identification and is attempted under a higher degree of uncertainty. Note that unlike in other fields, there is no clear meaning of “unknown knowns” (bottom left quadrant) in CEC analysis.
Source: Adapted from Stein (2012)
Characterizing and communicating risk associated with CEC generally requires an adaptive management approach (Aven and Bouder 2020) that reduces uncertainty with additional data collection over time (i.e., moving from the unknown-unknown quadrant to the known-known quadrant in Figure 1), but analyzing CEC in the environment generally takes the opposite path. First, existing targeted analysis methods are attempted to ensure that the substance is not already known (e.g., a manufactured substance that was previously undetected in the environment). Then, “suspect lists” may be developed using additional lines of evidence to assess whether the CEC is similar to an existing substance. Finally, as a last resort, non-target analysis (NTA) methods might be employed to localize the identity of the CEC.
This Fact Sheet is intended primarily for a technical audience (analytical chemists, biotechnologists, etc.) who might be involved in the development of programs for the analysis of previously unidentified CEC. It is accompanied by a glossary of technical terms and a case study that focuses on the identification of a previously unknown CEC. The overview of recent advances in analytical methods adoption and development covered in this Fact Sheet is presented for four categories of CEC: individual chemical compounds, chemical classes, biological contaminants, and particulates. Challenges to the development of analytical methods for these categories range widely from the absence of reference materials for identification and quantitation (e.g., individual chemicals and biological contaminants) to the lack of specificity (e.g., chemical class analysis and biological contaminant molecular primers) and unique categorization and characterization procedures (e.g., particulates and subclasses within chemical classes). Analytical approaches that address these challenges are described in the sections that follow.
2. Individual Chemical Compound Analysis
2.1 Individual Compound Identification
Investigation of high-priority CEC has grown in recent years due to advances in analytical methods, instrumentation, and data processing that make it possible to detect lower concentrations of more compounds in complex environmental samples. Common instruments used to analyze CEC include separation by liquid chromatography (LC) or gas chromatography (GC), and detection by tandem mass spectrometry (MS/MS) or high-resolution mass spectrometry (HRMS).
There are three approaches to detecting CEC in environmental samples (Figure 2 and Table 1): targeted analysis, suspect screening, and NTA.
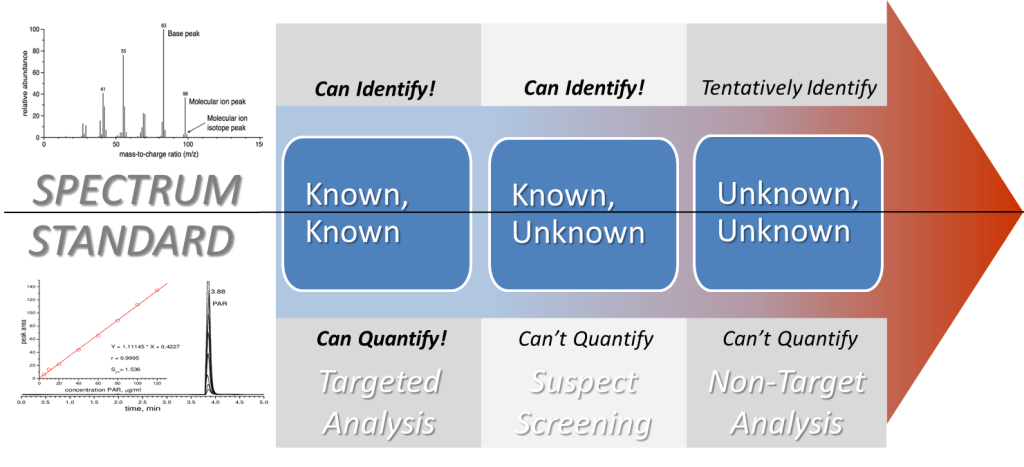
Figure 2. Targeted screening to non-target analysis. CEC that are known-knowns are compounds that are on target analyte lists and have established methods for detection and quantitation (targeted analysis). CEC that are known-unknowns are compounds included in a suspect list and can be identified but not quantified because a reference analytical standard is not available. CEC that are unknown-unknowns are compounds that were not included in a suspect list and cannot be quantified; experimental data must be used to elucidate a tentative chemical structure. Both known-unknowns (suspect screening) and unknown-unknowns (NTA) require HRMS for structural identification and confirmation.
Source: ITRC CEC Team.
Table 1. Targeted, suspect screening, and non-target analysis
Source: ITRC CEC Team.
| Targeted Analysis | Suspect Screening Analysis | Non-Target Analysis | |
| Description | “Known-Knowns” | “Known-Unknowns” | “Unknown-Unknowns” |
| Question | Is Compound X in the sample? At what concentration? | Which compounds from the list are in the sample? | Which compounds are in the sample? |
| Uses | Quantify known CEC | Identify likely compounds without standards | Identify any compounds without standards |
| Scale of Use | Widely used “Gold Standard” |
Moderate, primarily in research labs Minimal in commercial labs |
Moderate, primarily in research labs Minimal in commercial labs |
| Instrument | Tandem MS (MS/MS) | HRMS | HRMS |
| Before You Start | Analytical standards List of compounds prior to analysis Compound-specific methods | No standards required Suspect list after analysis Spectra library General method | See Suspect Screening Analysis Peak picking algorithms in silico spectral library |
| Resources | USEPA Environmental Sampling and Analytical Methods (ESAM) Program (ORD USEPA 2022) ISO methods (ISO 2023) | NORMAN Suspect Exchange USEPA Chemical Dashboard Confidence level identification BP4NTA | See Suspect Screening Analysis Highly skilled analyst |
| Benefits: | Quantitative Standardized QA/QC Well defined scope Established analytical methods Highly selective and sensitive High analytical accuracy and precision Matrix-specific reference material available | Broad scope Defined list of compounds No standards required General method Data can be reanalyzed later | See Suspect Screening Analysis Undefined scope |
| Uncertainty/ Challenges | Multiple methods required Narrow scope Expensive standards Method development for each compound Cannot reprocess data | Qualitative (presence or absence) Limited by database and spectral library Defining confidence level Limited QA/QC available Expensive instrumentation Less sensitivity than targeted methods | See Suspect Screening Analysis Communicating uncertainty in identification |
| Resources | SW-846 Style Guide (USEPA 2012) ISO 17025 (ISO 2017) ASTM International D3975-93 (ASTM International 2023) | Chemical database Spectral library BP4NTA Schymanski confidence scale (Schymanski et al. 2014) | See Suspect Screening Analysis |
| When to Use This | Monitoring Program for Specific CEC Example: 1,4-dioxane in drinking water Approach: Use 1,4-dioxane method | Suspect CEC occurrence Example: known AFFF release Approach: use of comprehensive PFAS suspect list to match data to named compounds from list. | Unknown CEC occurrence Example: industrial release of unknown compound Approach: find features and identify using databases, in silico spectral libraries, homologous series, and characteristic fragments and evaluate importance using known or surrogate toxicity data. |
Notes: AFFF = aqueous film–forming foams, BP4NTA = Benchmarking and Publications for Non-targeted Analysis, HRMS = high-resolution mass spectrometry, ISO = International Organization for Standardization, MS = mass spectrometry, NORMAN = network of reference laboratories, research centers, and related organizations for monitoring of emerging environmental substances, PFAS = perfluoroalkyl and polyfluoroalkyl substances, QA/QC = quality assurance / quality control, USEPA = United States Environmental Protection Agency.
2.1.1 Targeted Analysis: “Known-Knowns”
Is compound X in the sample and at what concentration?
Targeted analysis is used when (1) a CEC is identified prior to analysis, (2) there is an established analytical method and (3) analytical standards are available. Targeted analysis uses MS/MS to identify and quantify target compounds. It is the current “gold standard” of CEC detection and quantitation. It is highly selective and sensitive, has high analytical accuracy and precision, and includes a well-defined scope. Best practices for developing targeted methods can be found in many sources including, but not limited to, peer-reviewed journal articles, the SW-846 Method Style Guide (SW-846 Style Guide, 2012) (USEPA 2012), International Organization for Standardization (ISO) 17025 (ISO 17025) (ISO 2017), or the ASTM International D3975-93 (ASTM International 2023).
Targeted analysis relies on available reference material, analytical standards, and established analytical methods. If the CEC is not known or a standard is not currently available, targeted screening cannot be performed. Targeted methods are specific to a select set of compounds. For a complete CEC characterization, a full suite of targeted methods may be performed to capture the widest range of possible chemistries of the potential CEC.
2.1.2 Suspect Screening: “Known-Unknowns”
Which compounds from the list are in the sample?
Suspect screening is a qualitative analysis. It is an evolving analytical technique that aims to identify compounds that are expected to be present in the sample (e.g., based on a user-generated list of past chemical uses and releases at a site) without using reference or analytical standards. Suspect screening requires HRMS and the use of chemical databases, prioritization lists, and spectral libraries (when available) or in silico structural prediction algorithms to identify compounds and determine the confidence and uncertainty in the match. Suspect screening lists are user defined and may be tailored to a specific site (e.g., wastewater treatment plant), situation (e.g., chemical spill), or concern (e.g., forensic analysis of illicit drugs). The user-defined list must contain analytes that are relevant to the analytical instrument chosen for analysis. For example, nonvolatile compounds such as pharmaceuticals, personal care products, or PFAS are more amenable to liquid chromatography–mass spectrometry (LC-MS) analysis whereas volatile compounds, such as solvents, some pesticides, and flame retardants are more amenable to gas chromatography–mass spectrometry (GC-MS) analysis and may be captured as tentatively identified compounds (TICs) when high-quality mass spectra are available. The user-defined lists will require updates as needed.
Spectral libraries, when available, provide an additional layer of confidence to the identification of an unknown molecular feature (i.e., a peak with a specified mass to charge ratio (m/z), retention time, and area). Volatile compound analysis employs GC-MS with high-energy electron impact (EI) ionization at 70 eV (electron volts) as the standard approach. EI produces highly reproducible fragments, or “fingerprints,” which are comparable across different laboratories and instrument manufacturer platforms. Therefore, experimental fragments can be screened against a curated spectral library of authentic chemical standards. If an authentic chemical standard is not available, the feature may be assigned a similarity or “match” score based on another structurally similar compound. Database or spectral libraries that are commercially available are curated, maintained, and updated on a regular basis. A spectral library may contain anywhere from 10,000 to more than 750,000 compounds collected by GC-EI-MS and may be used for spectral interpretation.
Analysis of nonvolatile or semi-volatile compounds by LC-MS is not standardized because a compound class may require optimization of the ionization mode, polarity, ionization energy, or collision energies. MS method conditions or fragments can be specific to the laboratory or instrument manufacturer platform. Many LC-MS spectral libraries are open-source repositories that allow public sharing of reference mass spectra and provide the MS conditions so that results can be replicated. These spectral libraries may be user generated, uncurated, poorly annotated, or still under development. For these reasons, many LC-MS users prefer to create and use their own spectral libraries and perform manually or in silico MS fragment interpretation when reference spectra are not available. Resources are identified in Table 1. Suspect screening may provide a more comprehensive result of which compounds are in the sample; however, it is not quantitative, and the scope may be undefined.
2.1.3 Non-Target Analysis: “Unknown-Unknowns”
What is in the sample?
Non-target analysis (NTA) is another qualitative analysis. It is an evolving analytical technique that uses many of the same tools as suspect screening (see above for information regarding suspect lists and spectral libraries) but aims to identify as many compounds as possible in a sample. NTA relies on algorithms to select important features in the data, compare them to available databases and spectral libraries, and prioritize those features in the data by occurrence or available toxicity data (experimental or predicted) to make conclusions about the results.
At the time of writing, there are no standardized methods for suspect screening and NTA, but advances in study design (Hollender et al. 2017), quality assurance/quality control (QA/QC) metrics (Peter et al. 2021), and Open and FAIR (Findability, Accessibility, Interoperability, Reusability) databases (Mohammed Taha et al. 2022) are developing rapidly (Schymanski and Bolton 2021). Two main challenges with suspect screening and NTA include standardizing QA/QC metrics and developing common language around identification confidence.
Benchmarking and Publications for Non-Targeted Analysis (BP4NTA) has developed a rigorous QA/QC framework to evaluate performance criteria such as study design, data acquisition, data processing and analysis, data outputs, and QA/QC metrics (Peter et al. 2021). The Study Reporting Tool (SRT) is an open-source guide for reporting NTA data. It provides a framework for NTA design, data communication, and reporting performance metrics to ensure gathering and interpreting high quality data.
The other main challenge to NTA is communicating the level of confidence and, conversely, uncertainty in the compound identification. The confidence levels developed by Schymanski and colleagues in 2014 for characterizing uncertainty in structural identification (Schymanski et al. 2014) are widely used to assess the evidence gathered by HRMS analysis (Figure 3). For example, if an authentic chemical standard is used to verify the CEC, then it would have Level 1 Identification Confidence. If an authentic standard is not available, but a chemical database and spectral libraries can provide supporting evidence for molecular formula and molecular structure, then it would have Level 2 Identification Confidence. A Level 3 identification would include a CEC with descriptive spectral information that could be used to assign a tentative structure to the CEC. Level 4 identification would only include a chemical formula with no structural information, and a Level 5 identification would only contain an exact mass with no chemical formula or structural information. Charbonnet and colleagues modified the Schymanski scale for PFAS (Charbonnet et al. 2022) by including the presence of homologues and characteristic fragments when additional information, like a spectral library match, is not available.
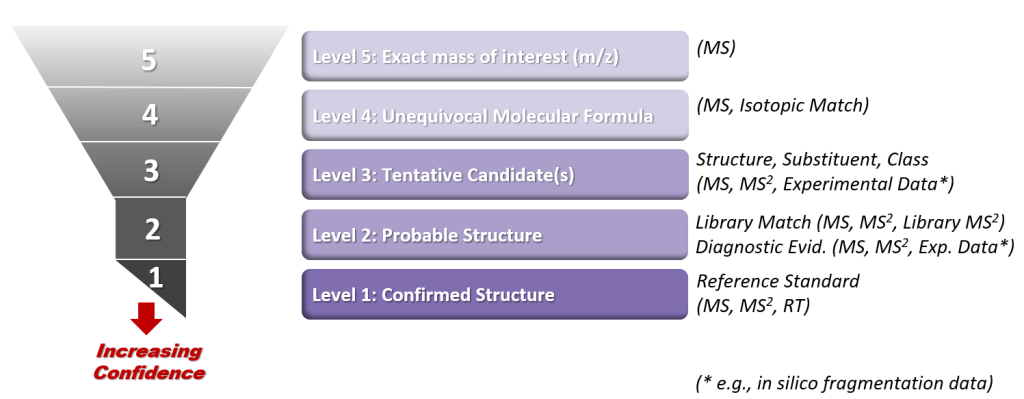
Figure 3. Schymanski scale. The Schymanski Confidence Levels are used for categorizing uncertainty in the identification of small chemical molecules using HRMS. Levels 5 and 4 rely primarily on the HRMS molecular ion peak (MS). Levels 3 and 2 use MS fragmentation data (MS2) and experimental information as well. Level 1 identification relies on all of the prior information and a confirmation (retention time and spectra) using a reference standard.
Source: Adapted from Schymanski and colleagues (2014).
These approaches can be used in parallel to assess risk, characterize CEC, and make more informed decisions. At the time of writing, NTA is mainly performed in academic and government research laboratories and research and development specialty projects because the analysis requires specialized equipment, highly trained analysts, and time-intensive data processing. Developments in databases and libraries, data processing, information sharing, and communication have created an opportunity for NTA methods for detecting CEC to become more widespread.
2.2 Resources
The lists below provide examples of where to find information on methods, lists, and spectral data.
- Where to find methods?
- United States Environmental Protection Agency (USEPA) Selected Analytical Methods (SAM) for Environmental Remediation and Recovery, 2022, Appendix A, https://www.epa.gov/esam/selected-analytical-methods-environmental-remediation-and-recovery-sam-2022 (ORD USEPA 2022)
- ISO (ISO 2023)
- National Institute for Occupational Safety and Health (NIOSH 2003)
- Occupational Safety and Health Administration (OSHA) (DOL 2023)
- National Environmental Monitoring Index (NEMI): https://www.nemi.gov/home/ (USGS 2023)
- Where to find lists?
- CompTox Chemicals Dashboard: https://comptox.epa.gov/dashboard/chemical-lists (ORD USEPA 2023)
- NORMAN Network: https://www.norman-network.com/?q=suspect-list-exchange (Mohammed Taha et al. 2022)
- PFAS Suspect Lists: NIST https://github.com/usnistgov/NISTPFAS (Place 2023)
- ChemSpider: http://www.chemspider.com/ (Royal Society of Chemistry 2023)
- PubChem: https://pubchem.ncbi.nlm.nih.gov/ (PubChem 2023)
- TSCA: https://www.epa.gov/tsca-inventory (OCSPP USEPA 2014)
- NIST Chemistry WebBook: https://webbook.nist.gov/chemistry/ (DOC 2023)
- Where to find spectral data?
- Experimental data:
- MassBank: https://massbank.eu (MassBank-Consortium 2021)
- MoNA: https://massbank.us (MoNA 2023)
- mzCloud: https://www.mzcloud.org (mzCloud 2023)
- NIST Tandem Mass Spectral Library: https://www.nist.gov/programs-projects/tandem-mass-spectral-library (NIST 2012)
- in silico spectral data
- SIRIUS+CSI:FingerID: https://bio.informatik.uni-jena.de/ (Lehrstuhl Bioinformatik Jena 2023)
- BioTransformer: http://biotransformer.ca/ (Djoumbou-Feunang et al. 2019)
- MetFrag: https://msbi.ipb-halle.de/MetFragBeta/ (Ruttkies et al. 2016)
- Experimental data:
3. Analytical Methods For Chemical Classes
3.1 Why Chemical Class Analysis?
A contaminant chemical class can be defined structurally, as in the case of PFAS (ITRC 2018), leading to a theoretical possibility of thousands of compounds (OECD 2018). Correspondingly, a chemical class can also be defined operationally as in the case of disinfection byproducts (DBPs), where any organic or inorganic compound formed during disinfection is a DBP, thereby leading to near infinite structural possibilities of its members (Dong, Cuthbertson, and Richardson 2020) (Dong et al. 2020). For such chemical classes, targeted analysis is limited to the few compounds for which reference analytical standards are commercially available, leaving the threat posed by the remining fraction unknown. Consequently, chemical class analysis is needed to assess the full scope of the problem, especially when toxicity is perceived to be similar across the chemical class.
The main characteristics of chemical class analysis include the following: (1) the class consists of a considerable number of members; (2) the specific identities of the majority of its members are unknown; and (3) a key property of the chemical class can be exploited to develop a class- or subclass-wide analytical method. A major challenge in the development and use of chemical class methods is the exclusion of interfering compounds outside the chemical class. This specificity problem is prevalent in chemical class methods, especially since the identities of the vast majority of its members are unknown. To illustrate the challenges associated with chemical class analysis, we present the case of total organofluorine and total PFAS analysis below.
3.2 Example of Chemical Class Analysis: Organofluorines and PFAS
In recent years, there has been heightened interest in organofluorine analysis because of the persistence of PFAS in environmental media and their widespread use in commercial products (Robel et al. 2017). PFAS rarely occur in the environment as isolated individual compounds and occur more commonly as mixtures (Bălan et al. 2021). According to the latest estimates, somewhere between 4,730 (2018)(OECD 2018) and 6,330 (Bălan et al. 2021) different PFAS compounds have been commercially produced to date. Broadly speaking, PFAS can be divided into four different categories: perfluoroalkyl acids (PFAAs), PFAA precursors, perfluoropolyethers (PFPEs), and fluoropolymers (Buck et al. 2011). Although PFAS consist of a wide universe of functionally different substances, there is a dynamic nature to PFAS mixtures in the environment because many PFAS (other than PFAAs) act as precursors and transform to PFAA terminal products over time (Bălan et al. 2021).
To date, targeted analytical methods have focused on a limited number of PFAS, with a hard limit of less than 100 commercially available analytical reference standards for the identification and quantitation of these compounds (ITRC 2023a; McDonough, Guelfo, and Higgins 2019). This leaves the vast majority of PFAS in environmental media as an unknown fraction that cannot be evaluated further without methods that target PFAS and organofluorines more generally as a chemical class. A variety of methods ranging from total fluorine analysis to PFAS organofluorine analysis have been attempted to characterize the unknown fraction. The relationships among the most common total and PFAS organofluorine methods are depicted in Figure 4 and summarized in Table 2. The reader is directed to the Interstate Technology and Regulatory Council (ITRC) PFAS Committee product Section 11 (Sampling and Analysis Methods) for further details on most of these methods (ITRC 2023a). Section 11 details published chemical class and targeted analysis methods for PFAS.

Figure 4. The relationships among total fluorine, organofluorine, PFAS organofluorine, and targeted PFAS methods.
Source: ITRC CEC Team.
Table 2. Summary of major methods used for the analysis of total fluorine, organofluorine, and PFAS organofluorine
| Method | Chemical Class/ Subclass Targeted | Principle | Destructive/ Nondestructive to Sample | Environmental Matrices Analyzed | Sensitivity | Limitations | Key References |
| Particle-Induced Gamma Ray Emission (PIGE) | Total fluorine (organic + inorganic) | An accelerated beam of protons strikes the surface of the sample of interest, exciting 19F nuclei. γ-rays emitted upon de-excitation provide a unique signature proportional to the number of fluorine atoms on the surface. Technique has been applied to textile-based standards. | Nondestructive | Consumer products | 13–45 nmol-F/cm2 (~ 0.1 µg-F/L) | Surface-based technique with limited penetration depth (˂0.22 mm). Includes inorganic fluoride. | (Ritter et al. 2017) |
| Adsorbable/ Extractable Organic Fluorine (AOF/EOF) via Combustion Ion Chromatography (CIC) | Organic fluorine | AOF uses a polystyrene divinylbenzene-based solid phase while EOF uses a customizable ion-pairing solid phase extraction of samples. The extract is then eluted and analyzed via combustion ion chromatography (CIC). | Destructive | AOF: natural waters; wastewater. EOF: biological materials; sediments, soils, and sludge; natural waters; wastewater |
≤ 1 µg-F/L | Influenced by choice of sample preparation approach for isolation of the organofluorine-containing fraction. Can include non-PFAS compounds such as fluorinated pharmaceuticals and pesticides. EOF has yet to be standardized. | AOF: (Wagner et al. 2013; OW USEPA 2022; Willach, Brauch, and Lange 2016) EOF: (Miyake et al. 2007; Kärrman et al. 2021) |
| Moody 19F Nuclear Magnetic Resonance | PFAS organic fluorine | Monitors chemical shift associated with the terminal CF3 peak, thereby selecting mainly for fluorinated surfactants and eliminating most interferences from common classes of organofluorine pesticides or pharmaceuticals, as well as inorganic fluoride. | Nondestructive | Natural waters, wastewater | ≥ 1 µg-F/L (this technique selects for fluorinated PFAS surfactants) | Relatively high detection limits in aqueous samples. | (Moody et al. 2001) |
| Total Oxidizable Precursor (TOP) | PFAS organic fluorine | Oxidizes “precursor” molecules using an excess of hydroxyl radicals. The resulting predominantly PFAA fraction is then target screened using LC-MS/MS. | Destructive | Consumer products; sediments, soils, and sludge; natural waters; wastewater | 0.1–0.5 ng/L for individual PFAS | Oxidation products analyzed via targeted analysis for PFAAs and, therefore, limited by the available reference standards for PFAAs. PFAS that are not PFAA precursors are not measured by this assay. | (Houtz et al. 2013; Houtz and Sedlak 2012) |
Notes: LC-MS/MS = liquid chromatography–tandem mass spectrometry, µg-F/L = microgram fluorine per liter, mm = millimeters, ng/L = nanogram per liter, nmol-F/cm2 = nanomole fluorine per square centimeter, PFAA = perfluoroalkyl acids, PFAS = perfluoroalkyl and polyfluoroalkyl substances
3.3 Online Resources
Sample online resources are listed below:
- ITRC PFAS Section 11.2, Analytical Methods and Techniques, https://pfas-1.itrcweb.org/11-sampling-and-analytical-methods/ (ITRC 2023a)
- USEPA Draft Method 1621, Screening Method for the Determination of AOF in Aqueous Matrices by CIC, https://www.epa.gov/system/files/documents/2022-04/draft-method-1621-for-screening-aof-in-aqueous-matrices-by-cic_0.pdf, (OW USEPA 2022)
- USEPA, Frequent Questions about PFAS Methods for National Pollutant Discharge Elimination System Permits (NPDES), https://www.epa.gov/cwa-methods/frequent-questions-about-pfas-methods-npdes-permits (OW USEPA 2020)
4. METHODS FOR BIOLOGICAL CONTAMINANTS
4.1 Survey of Microbial Pathogens That Are Established or Emerging
There are established lists of known microbial pathogens that are reliably detected by a variety of molecular biological, culture-based, or analytical methods. These lists are published by the Food and Drug Administration (FDA), Centers for Disease Control (CDC), and other regulatory agencies globally. Some examples are listed below:
- In food: https://www.fda.gov/media/83271/download (FDA 2012) and https://www.fsis.usda.gov/food-safety/foodborne-illness-and-disease/illnesses-and-pathogens (USDA 2020)
- Various sources: https://wwwnc.cdc.gov/eid/ (CDC 2022) and https://www.cdc.gov/ncezid/index.html (CDC 2023b)
- MicrobNet: https://www.cdc.gov/amd/what-we-do/emerging-threats.html (CDC 2019) and https://microbenet.cdc.gov/ (CDC 2023a); new pathogens are added to this database monthly, and it can be searched by organism name, or for unknowns, search by 16S sequence results (biochemical test results), phenotypic results, or from matrix assisted laser desorption or ionization–time of flight (MALDI-TOF) data
- EMERGE: Efficient response to highly dangerous and emerging pathogens at the European Union level, https://www.emerge.rki.eu/Emerge (Robert Koch Institute 2023); 40 diagnostic laboratories focused on risk group 3 bacteria and risk group 3 and 4 viruses
- GWPP: Global Water Pathogen Network, https://www.waterpathogens.org/ (GWPP 2015); pathogens impacting sanitation and safe water
Emerging concerns include reliably identifying previously unknown organisms that present public health impacts before they become a public health concern.
4.2 Microorganism Detection and Quantification Methods in Environmental Media
Detection and enumeration of emerging pathogens in water, wastewater, and/or environmental matrices (e.g., indoor or outdoor air, soil, surfaces) can be done by multiple different molecular biology–based or culture-based techniques. Many of these techniques were originally developed for applications in medicine, industry, agriculture, or defense. A prior ITRC group, Environmental Molecular Diagnostics (EMD) (ITRC 2013c), discussed a myriad of methods for detection and quantitation of microorganisms of importance for environmental remediation applications. Many of the methods described in the EMD guidance document could be and have been modified for the detection of emerging pathogens. For example, the gold standard method for detecting SARS-CoV-2 shed by individuals ill with COVID-19 in wastewater is quantitative polymerase chain reaction (qPCR). The detailed description of qPCR and other molecular biology–based methods are described in the EMD guidance. These methods are described briefly in Table 3 below with links to the pertinent information on the ITRC website. Additional techniques available for emerging pathogen detection (e.g., new “omics”-based techniques) are also presented in Table 3. Many of these “omics” techniques, such as metagenomics, metatranscriptomics, metabolomics, and proteomics, are described further in the table below and are defined in the glossary.
Table 3. Detection methods for emerging pathogens
Source: CEC Team.
| Method | Brief Description | Advantages for Detecting Emerging Pathogens | Disadvantages for Detecting Emerging Pathogens | Example Use for Emerging Pathogen Detection |
| Polymerase Chain Reaction (PCR) (ITRC 2013b) | Detects pathogenic microorganism nucleic acids (ITRC 2013b) or genes in sample. Provides a direct line of evidence of the presence of a pathogen. |
|
|
Detection of polio and monkeypox in wastewater |
| Real-Time Quantitative Polymerase Chain Reaction (qPCR) (ITRC 2013a) or Reverse Transcriptase qPCR (RT-qPCR) for RNA | Quantifies the abundance and/or activity of a target pathogenic microorganism through detection of nucleic acids or genes (ITRC 2013b) in a sample. This provides a direct line of evidence of the presence of a pathogen. |
|
|
Detection of SARS-CoV-2, polio, and monkeypox in wastewater Clinical gastroenteritis and antimicrobial-resistant qPCR array cards commercially available such as those from GenPix and Biomerieux |
| Genomics or Metagenomics | Analysis of genome of multiple organisms by next-generation sequencing (NGS) methods (N. Li et al. 2021; Loman et al. 2013) or complete analysis of the genome of a single pathogen. |
|
|
Investigate outbreaks of Escherichia coli strain O104:H4 in food supply (Loman et al. 2013) |
| Meta-transcriptomics | Analysis of the active genes in a pathogen or mixed sample of microorganisms (i.e., a virulence gene of E. coli) by NGS methods. |
|
|
Identification of vector- (i.e., mosquito) borne viruses (Batson et al. 2021) |
| Metabolomics | Mass spectroscopy technique to identify the suite of metabolites that are generated by microorganisms or pathogens. |
|
|
Nasopharyngeal swab samples identify acute respiratory illness caused by influenza (Hogan et al. 2021) Used for food safety monitoring (S. Li et al. 2021; Jadhav et al. 2018) |
| Proteomics | Mass spectrometry to identify the protein expression of pathogens. |
|
|
Species-level detection of foodborne pathogens (e.g., Listeria, Salmonella, and E. coli) (Syu, Dunn, and Zhu 2020) |
| Microarrays | Massively parallel detection of functional pathogen proteins or nucleic acids of pathogens. |
|
|
Functional protein arrays for zika and dengue fever (Savidis et al. 2016) |
| Flow Cytometry | A type of automated fluorescence detection technique allowing for detection of cells in complex matrices. |
|
|
Viral pathogen detection in treated wastewater for potable reuse (Rockey et al. 2019) |
| Culture-Based Methods | Growth of organisms in selective media specific to pathogen. Isolation of the pathogen may be required. |
|
|
FDA methods (FDA 2023) for detection of pathogens in food |
Metagenomics is the analysis of the genome (complete DNA or RNA sequence) of one or more organisms. In environmental surveillance for emerging pathogens, metagenomics can be used to identify the genome of new organisms within a community of interest (e.g., sputum or serum from ill individuals or environmental samples suspected of harboring human pathogen) (N. Li et al. 2021). This method allows for identification of suspected pathogens from among the complex microbial communities. Additional methods will be required to conclusively link any suspected genome identified in a sample to a particular morbidity or disease. Bioinformatics is then leveraged to identify pathogens from among the mixed community sequences. Therefore, if a novel pathogen is sequenced for which there are few similar sequences in the databases used for bioinformatic comparison, it will be difficult to impossible to determine which organism may be responsible for the disease.
Metatranscriptomics is similar to metagenomics in the way it depends on sequencing of nucleic acids and bioinformatics. The methods differ in that metatranscriptomics is targeted to sequencing genes that are currently being expressed in an organism—either a pathogen or a host organism (e.g., human). For example, genes associated with immune responses to respiratory viruses (e.g., interferon response genes and chemokines) can be sequenced from the host and compared to databases of known metabolic genes in humans (Rajagopala et al. 2021), such as those compiled in the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa et al. 2023).
Metabolomics harnesses the investigation of the organic compounds in samples (i.e., water or biological samples) that arise from metabolic activity of pathogens that are present in a sample or that are infecting a living organism. Some of the organic compounds metabolized by living organisms are volatile and can be detected by mass spectroscopy. Specifically, volatile organic compounds (VOCs) can be detected from pathogen isolates, expired human breath, sputum, feces, saliva, throat swabs, and vaginal discharge. Detection of these VOCs or biomarkers are an indicator of active infection and/or the presence of the pathogen in a sample. While routine microbiology surveillance for a known pathogen is time-consuming, the response in humans to a particular disease state may be a suitable and rapid marker of the presence of a pathogen (i.e., detection of VOCs in the breath of individuals with cystic fibrosis infected with Pseudomonas aeruginosa) (Gilligan 2021). Other examples of the use of metabolomics include the detection of Salmonella in milk via testing of exogenous VOCs (Bahroun et al. 2018).
Protein function is representative of the entirety of active biological processes in a cell, tissue, or bodily fluid. While metatranscriptomics measures the RNA that serves as intermediates between the genome and the protein, RNA is only an approximation of the levels of a protein that are in an infected host or a pathogen. The term proteome refers to the entire complement of proteins that can be made by a genome, while proteomics refers to the analysis of those proteins. By analyzing proteins in a microbial community or in an infected host, it is possible to identify groups of pathogens. However, additional tools are needed to conclusively determine the identity of novel pathogens (Bostanci et al. 2021). Biotyping of bacteria by proteomics is used in clinical laboratories, but is not typically applied for viruses (Grossegesse et al. 2020).
4.3 New Pathogen Detection Method Validation Approaches
Once an emerging pathogen has been identified as a causative agent of a disease, robust, rapid, reproducible, and ideally inexpensive detection methods are required. Robustness of the method here refers to the ability to detect the pathogen among many inhibitory compounds and/or confounding microorganisms or signals. Ideally, the analytical method would be rapid, and results would be obtained in near real time or within minutes of sample collection. Reproducibility of the method would occur if many different laboratories and/or analysts could obtain the same results within an acceptable margin of error on identical samples. The ideal method would be inexpensive as potentially tens, hundreds, or thousands of samples would need to be processed during a suspected public health outbreak or investigation of a site.
Analytical methods for pathogens would ideally have the following characteristics, in decreasing order of importance:
- quantitation (preferred) of viable and infectious pathogens (i.e., counts of cells)
- detection of markers (less preferred) of the pathogen (e.g., proteins or nucleic acids such as DNA)
- rapid quantitation or detection of pathogen (i.e., real-time results or within minutes of sample collection)
- sensitive detection of the pathogen in mixed matrices and from among mixtures of microorganisms
- reproducible method among many laboratories
- conducted with readily available and common equipment or test kits
Various guidance documents are available from federal agencies that recommend validation approaches for analytical methods. These references include, among others, the following:
- FDA guidance for validation of analytical methods for microbial pathogens in food and feed, https://www.fda.gov/media/83812/download (FDA 2019)
- MIQE (minimum information for publication of qPCR experiments) https://pubmed.ncbi.nlm.nih.gov/19246619/ (Bustin et al. 2009)
- USEPA QA/QC for labs running qPCR, https://projects.itrcweb.org/emd-2/Content/Resources/EPA-815-B-04-001.pdf (USEPA 2004)
- USEPA method validation and peer-review guidelines: Microbiological Methods of Analysis https://www.epa.gov/measurements-modeling/method-validation-and-peer-review-policies-and-guidelines (USEPA 2016)
A summary of the advantages and drawbacks to various methods of pathogen identification and detection has been presented previously and should also be referenced when considering methods for identification of emerging pathogens (Rajapaksha et al. 2019).
5. Analysis of Particulates
5.1 Microplastics
The ITRC Microplastics Team Materials (ITRC 2023b) details sampling and analysis techniques for microplastics (MPs).
5.1.1 Sampling
Techniques and best practices for sample collection and analysis of MPs and fibers are still evolving. In 2020, ASTM International adopted a standard for water sample collection, which covers low, medium, and high ranges of suspended solids (ASTM International 2020).
Publications on appropriate study data quality objectives (DQOs) for monitoring programs exist (OCSPP USEPA 2023) and could include the following:
- identification and determination of mass of MP
- identification of particle number, size, and shape of MP
- characterization of specific properties of individual MP
- polymer type
For general monitoring, the size of the MP should be defined by the program, with the understanding that the smaller sizes are more difficult (i.e., more costly) to sample, extract, and identify.
5.1.2 Analysis
Chemical identification/compositional analysis of the particles is critical for accurate quantification of MPs in environmental samples and is challenging because MPs can mimic naturally occurring materials and vice-versa. Visual microscopic methods are generally less than 70% accurate (Lenz et al. 2015) for larger fractions of MPs but far less effective for smaller MP fragments. Instrumentation allows not only for the identification of specific polymers of MPs, but also for the identification of other contaminants and adsorbates associated with the MPs. Detection approaches can be divided into the following categories:
- Visual Methods: Visual examination of a sample with or without magnification.
- Spectroscopic Methods: Capture and assign the characteristics of specific chemical structure of polymers using reference spectra (e.g., Fourier Transform Infrared (FTIR) microscopy and Raman Spectroscopy).
- Thermoanalytical/Chemical Methods: Pyrolyze the sample under inert conditions and detect specific decomposition products of the individual polymers (e.g., Pyrolysis GC-MS and Thermal Desorption GC-MS).
Applicable standards that discuss analysis include Raman and FTIR microscopy methods for MP identification in drinking water. This standard was adopted by the California State Water Board (Wong 2021).
Instrumentation methods are well summarized in the draft ITRC guide on MPs and are reproduced here in Table 4 (ITRC 2023b). The general workflow for selecting analysis and measurement methods based on the target and type of analysis is presented in Figure 5.
Table 4. Summary of microplastics characterization techniques
Source: ITRC 2022b.
| Description | Analysis Time/ Sample | Size Detection Limit | Measurement Preparation | Identifies Polymer Types | Detects Additives/ Surface Chemicals | Detects Particles or Mass |
| Visual Methods | ||||||
| NE Naked Eye |
Hours | 1 mm | None | No | No | Particle |
| SM Stereo Microscopy |
Hours | 100 µm | On filter | No | No | Particles |
| FM Fluorescence Microscopy |
Hours | 50 µm (possibly smaller based on objective lens used) |
On filter | No | No | Particles |
| SEM Scanning Electron Microscopy |
Hours | 0.001 µm | On filter | Yes | No | Particles |
| Spectroscopic Methods | ||||||
| FPA-FTIR Focal Plane Array-Fourier Transform Infrared Spectroscopy (in Transmission Mode) |
Hours | 20 µm | On special filter | Yes | No | Particles |
| FTIR Fourier Transform Infrared Spectroscopy (in Transmission Mode) |
Days | 20 µm | On special filter | Yes | No | Particles |
| LDIR Laser Direct Infrared Spectroscopy |
Minutes particles/ hour | 20 µm | Special microscope slide | Yes | No | Particles |
| NIR, vizNIR Near Infrared Spectroscopy, Visible-Near Infrared Spectroscopy |
Hours | Unspecified | On filter | Yes | Surface chemicals only | Particles |
| Raman Spectroscopy |
Days | 1 µm (theoretically but challenging to achieve) |
Extraction and placed on filter | All polymers | Yes | Particles |
| Thermoanalytical/Chemical Methods | ||||||
| DSC+TGA Differential Scanning Calorimetry+ Thermal gravimetric Analysis |
Hours | Unspecified | Filtrate | Yes, only PE, PP | No | Mass |
| Py-GC/MS Pyrolysis–Gas Chromatography–Mass Spectrometry |
Hours | <1–0.5 µg | Isolated particles | Yes | Yes | Mass |
Notes: µg = microgram, µm = micrometer, mm = millimeter, PE = polyethylene, PP = polypropylene

Figure 5. Workflow for selecting an analysis and measurement method for microplastics.
Source: Adapted from Shimadzu (2020).
5.2 Engineered Nanoparticles
As technology evolves toward the nanoscale, the long-term effects on human health and ecosystem impacts are yet to be seen. This aspect of nanotechnology is a growing concern. The release of engineered nanomaterials (ENMs) and engineered nanoparticles (ENPs) into the environment is inevitable, but information on their long-term effects on plant life, human health, and the ecosystem is in short supply and often conflicting.
Accurate detection of ENPs in the environment is imperative to assess the risk posed to the environment and to human health. Establishing discharge levels from point/nonpoint sources of ENPs will not be possible unless there are reliable methods for identifying, characterizing, and measuring ENPs in environmental media. Traditional analytical technologies and systems need improvement in both sensitivity and specificity to various types of ENPs in a wide concentration range. Due to the nature and composition of ENPs, the sensitivity of traditional analytical methods is often not adequate to produce findings below the detection limit (false negatives).
ENPs vary in properties such as size, morphology, elemental composition, crystalline structure, etc.; therefore, it is essential to devise methods that detect nanoparticles in the environment by focusing on these properties. Small variations in these properties influence the bulk properties of nanoparticles, which in turn can have negative implications once end products are discharged into the environment. Therefore, multiple measurement and analytical techniques used to detect, quantify, and analyze nanoparticles have been developed. The methods have been grouped into different categories, as represented in Figure 6, based on their ability to determine and analyze distinct characterizing properties.

Figure 6. Analytical methods for characterizing engineered nanoparticles based on characterization property. See supplementary information table.
Source: ITRC CEC Team.
One strategy to obtain concentrated particles without changing the intrinsic properties of the ENPs is enrichment-separation-detection (Zhang et al. 2019). An oversimplified model for detecting, monitoring, and controlling ENPs is shown in Figure 7. The outlook and challenges for ENP enrichment-separation-detection are not fully developed due to a lack of standardization of methods for broad-spectrum detection. Each step is its own isolated process, which can introduce error during measurement. This can be mitigated if a rapid, fully integrated device is developed, but such a device is currently absent in analytical technologies.

Figure 7. Overview of detecting, monitoring, and controlling ENPs in an aquatic environment.
Source:Adapted from Zhang and colleagues (2019).
Some of the more commonly used qualitative and quantitative analysis techniques for ENPs are summarized below:
5.2.1 Single Particle Inductively Coupled Plasma–Mass Spectrometry
Single particle inductively coupled plasma–mass spectrometry (SP ICP-MS) was developed to facilitate chemical characterization of individual nanoparticles and to differentiate those particles from dissolved ions. In SP ICP-MS, a dilute suspension of particles is introduced into the ICP. In the ICP a dense ion cloud is produced, which is then transferred into the mass spectrometer, where it is guided through the ion optics and mass separation device as a transient pulse of ions with a typical temporal duration between 300 and 500 microseconds (μs). If enough ions from an ENP are passed through the MS instrument, then the ENP-produced transient pulse of ions is registered as a signal spike in the time-trace of the mass spectrometer. By counting these ENP spikes, particle number concentrations (PNCs) of analyte ENPs can be determined. The amount of ion signal in each ENP-produced signal spike can be related to the amount (i.e., mass) of an element in the ENP. In SP ICP-MS, the signal is monitored at a higher time resolution than standard ICP-MS. This allows the discrimination between the ion plumes caused by the particle and the background analyte. The intensity of the ion plume is related to the mass of metal in the ENP (Heithmar 2011). It is used for measuring inorganic ENPs at low number concentrations (10²–10⁶ particles/milliliter).
5.2.2 Raman Spectroscopy (Surface Enhanced Raman Spectroscopy [SERS], Tip Enhanced Raman Spectroscopy [TERS], Plasmon Enhanced Raman Spectroscopy [PERS])
For many nanomaterials, Raman spectroscopy has become one of the go-to characterization methods. The main reason is that Raman spectroscopy can not only determine the composition of each nanomaterial, allowing it to be identified, but it can often determine the structural arrangement that distinguishes two different forms of the same type of nanomaterial. One example of this is its ability to distinguish between single-walled carbon nanotubes and multi-walled carbon nanotubes.
5.2.3 Dynamic Light Scattering
Dynamic light scattering (DLS) uses scattered light to measure the rate of diffusion of ENPs. It provides information about size distribution in terms of hydrodynamic diameter. DLS uses a laser beam that passes through a liquid suspension containing the analyte particles. They scatter the incident laser at different scattering angles. The Brownian motion of the detected particles induces the shift in light frequency, which varies with different particle sizes (Langevin et al. 2018). DLS is a useful tool for determining the hydrodynamic diameter and particle size distribution in suspension and for investigating colloidal properties of nanoparticles. It is used for size characterization of lipid nano-capsules in food samples (Yegin and Lamprecht 2006).
5.2.4 Nanoparticle Tracking
Nanoparticle tracking (NT) involves the application of intense laser light to illuminate free-diffusing particles to track their Brownian motion with monochrome imaging (Bhattacharjee 2016). Unlike DLS, NT can measure particle-by-particle size. NT provides individual particle intensity as well as motion videos (Hou et al. 2018). NT is better relative to DLS in detecting smaller aggregates (Hou et al. 2018). Setup parameters need to be adjusted carefully to obtain high accuracy.
5.2.5 Electrochemical Methods
“Nano-impact”–based electrochemical methods have shown great promise in detecting ENPs at the single-entity level. The method is based on the direct impact of individual particles on the electrode surface, which leads to current spikes as a function of time that can correlate to various characteristics of an ENP’s property (Neves et al. 2020). Quantitative information for ENP characteristics such as size, concentration, aggregation, and catalytic reactivity can be generated (Cheng and Compton 2014).
Case Study: Effect-Directed Nontarget Analysis Identifies 6PPD-q as Cause of Urban Runoff Mortality Syndrome
1. Introduction
This case study describes an effect-directed nontargeted analysis (NTA) experimental study (Tian et al. 2021) to identify the contaminant of emerging concern (CEC) responsible for acute mortality in coho salmon (Oncorhynchus kisutch) observed during runoff events in the Pacific Northwest (a.k.a. “urban runoff mortality syndrome” [URMS]). URMS occurs among adult coho salmon populations annually when they return to spawn in freshwater, especially to waters located in urbanized watersheds. Effect-directed analysis (EDA) is a strategy for environmental profiling of samples that combines sample fractionation schemes, toxic effect assays, and chemical analysis to identify toxic pollutants (Dong, Cuthbertson, and Richardson 2020). More recently, EDA has been paired with NTA using high-resolution mass spectrometry (HRMS) for chemical analysis to identify CEC in complex environmental samples (Dong, Cuthbertson, and Richardson 2020; Hollender et al. 2019). The case study begins with tire leachate (i.e., tread wear particle leachate [TWPL]) in the “known-unknowns” quadrant of the Rumsfeld matrix (see Figure 1, Analytical Methods Fact Sheet) after targeted analysis methods had been exhausted and after a chemical compositional similarity had been established among TWPL, roadway runoff, and URMS–associated waters (Peter et al. 2018).
2. Experimental Methodology and Results
The experimental design of the study relied upon three key tools for identifying the CEC:
- A fractionation scheme to simplify the complex TWPL mixture
- Acute exposure toxicity assays to screen TWPL fractions for their ability to induce mortality in juvenile coho salmon
- Ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS) for structural determination (i.e., by molecular formula determination using accurate mass—see Figure 1)
Acute toxicity assays were performed on juvenile coho salmon as a proxy for adult coho (Chow et al. 2019). The CEC was eventually determined to be a quinone oxidation byproduct of the tire antioxidant N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6PPD), or 6PPD-quinone (6PPD-q). Note that only a cursory description of follow-up work beyond the molecular formula determination (i.e., to elucidate the chemical structure of the CEC) is provided within this case study writeup. The subsections that follow describe various stages of the study, which are also graphically summarized in Figure 2.
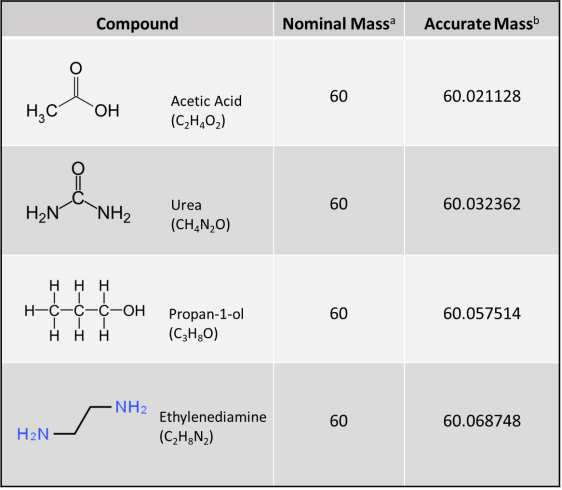
Figure 1. Accurate mass from HRMS for determining molecular formula. HRMS yields ion mass at an accuracy of three to seven decimal places (i.e., 0.001 Dalton [Da] [or amu] or less), thereby deciphering its unique molecular formula. A conventional unit‐resolution quadrupole mass spectrometer cannot distinguish the identity of a hypothetical 60 amu or Da (60 m/z) nominal mass molecular ion peak among four different compounds (i.e., acetic acid, urea, propan‐1‐ol, and ethylenediamine); however, a molecular ion peak of 60.0687 amu obtained using HRMS (e.g., by using a quadrupole‐time‐of‐flight mass spectrometer) would clearly indicate the compound ethylenediamine.
Source: ITRC CEC Team
a. Nominal mass is at unit resolution and is expressed as an integer. For monoisotopic ions (i.e., ions made up of only the most abundant isotope of each of its constituent elements), nominal masses of elements in atomic mass units (amu) or Daltons are C=12, H=1, N=14, and O=16.
b. Accurate mass is obtained from a high-resolution instrument that gives a molecular mass at an accuracy of three to seven decimal places. For monoisotopic ions, accurate masses of elements in amu or Daltons are C=12.000000, H=1.007825, N=14.003074, and O=15.994914.
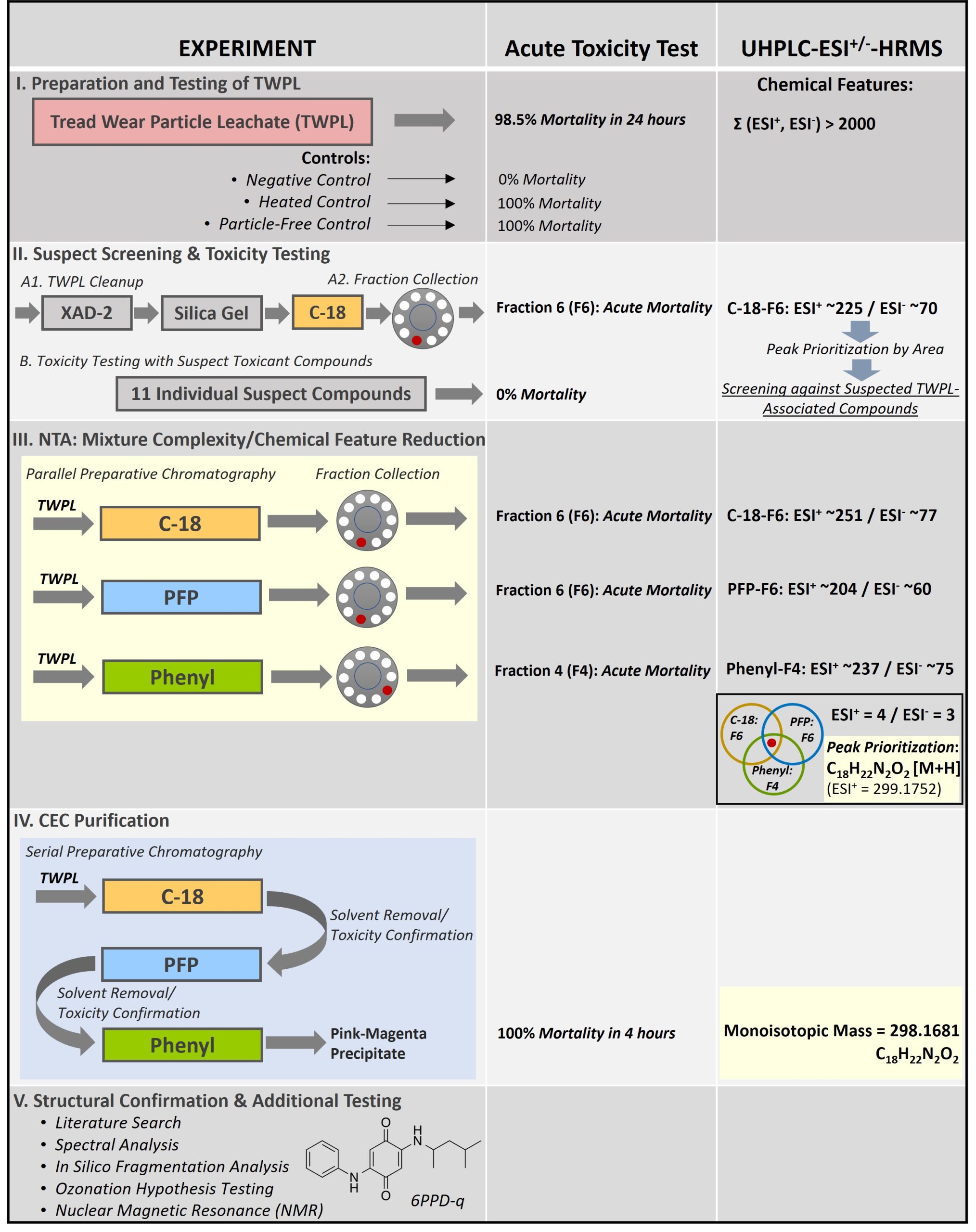
Figure 2. Overall schema for 6PPD‐q effect‐directed analysis (EDA) study
Source: Tian and colleagues (2021).
2.1 Stage I. Preparation and Testing of TWPL
An aqueous TWPL was prepared using tread particles from nine different used and new tires. The TWPL was found to be lethal (98.5% mortality in <6 hours) to juvenile coho salmon in a 24-hour acute toxicity test. A negative control demonstrated no mortality while toxicity remained unabated in a heated control, indicating stability during handling, and in a particle-free control, indicating that the CEC was dissolved and not particle-associated. Toxicity also remained unchanged after the leachate was treated with ion exchange resins (cation and anion) and ethylenediaminetetraacetic acid (EDTA) indicating that the CEC was not ionized and was not a metal, respectively. UHPLC-HRMS of the TWPL in positive and negative electrospray ionization modes (i.e., ESI+ and ESI–, respectively) produced more than 2,000 combined chemical features with unique mass to charge ratio (m/z) and retention time, indicating the complexity of the TWPL mixture. This necessitated cleanup of the mixture in Stage 2 to generate a simplified yet toxic mixture more amenable to individual compound identification.
2.2 Stage II. Suspect Screening and Individual Compound Toxicity Testing
In Stage II, the TWPL underwent sequential cleanup using cation exchange, silica gel, and reversed-phase C-18 separation. Fractions were collected and were subjected to acute toxicity testing. Only one fraction (Fraction 6 or F6) produced acute mortality in coho salmon, indicating that it contained the suspected CEC. The fraction was analyzed using UHPLC-HRMS, which resulted in approximately 225 and 70 chemical features in ESI+ and ESI– ionization modes, respectively. This constituted a significant reduction in sample complexity because only 10% of the features found in the initial TWPL mixture remained in the active fraction. A suspect screening list was compiled using an in-house database of tire rubber compounds (n=258) and a larger database from the NORMAN (network of reference laboratories, research centers and related organizations for monitoring of emerging environmental substances) Suspect List Exchange Database of approximately 30,000 environmental pollutants. These lists were integrated into the data-dependent acquisition to prioritize tandem mass spectral (MS/MS) collection for suspected compounds. Eleven compounds, including plasticizers, antioxidants, emulsifiers, and their transformation products, were identified in the active fraction but none induced mortality in the test organisms. The negative results from the suspect screening stage led the authors to conclude that the CEC responsible for URMS was previously unreported in scientific literature and required an NTA approach for identification.
2.3 Stage III. NTA: Mixture Complexity and Chemical Feature Reduction
A parallel preparative-level chromatography approach was employed to further reduce the mixture complexity and limit the number of chemical features. Columns with different stationary phases (C-18, pentafluorophenyl [PFP], and phenyl), together with fraction collection, were used to change the elution order of the CEC, as well as the mixture composition of each fraction. Only fractions C-18-F6, PFP-F6, and Phenyl-F4 induced acute mortality in test organisms. These toxic fractions were independently analyzed using UHPLC-HRMS and resulted in the ESI+ chemical features of 251 for C-18-F6, 204 for PFP-F6, and 237 for Phenyl-F4. Similarly, ESI– chemical features of 77 for C-18-F6, 60 for PFP-F6, and 75 for Phenyl-F4 were obtained. Common chemical features present in all three lethal fractions were identified, resulting in only four ESI+ and three ESI– chemical features.
Peaks for these common chemical features were prioritized by abundance, resulting in the identification of a dominant chemical feature (i.e., 10-fold higher intensity in both ESI+ and ESI– ) that that had a measured mass of 298.1681 atomic mass units (amu) (or ESI+ [M+H] protonated peaks of 299.1752–299.1777 amu). After subtracting the proton mass, this molecular ion peak corresponded to the monoisotopic ion (i.e., an ion composed of only the most abundant isotope of each element) with a molecular formula of C18H22N2O2. The structural information gleaned about the CEC at this stage would place it at Level 5 on the Schymanski Confidence Level Scale (see Figure 3, Analytical Methods Fact Sheet), where the exact mass of interest had been identified, but remained a high-priority CEC based on the toxicity results.
2.4 Stage IV. CEC Purification
The preparative chromatography setup in Stage III was converted from parallel to serial (i.e., C-18 PFP Phenyl) to recover a sufficient amount of material to facilitate chemical structure studies in Stage V. Solvent removal via centrifugal evaporation and toxicity confirmation between separations yielded a pink-magenta precipitate that was dominated by the C18H22N2O2 molecular ion peak via UHPLC-HRMS (chemical features: ESI+ = 4 and ESI‐ = 3). The material caused 100% mortality in juvenile coho salmon upon 4 hours of exposure.
2.5 Stage V. Structural Confirmation and Additional Testing
Various tools were employed to elucidate the structure of the CEC with a molecular formula of C18H22N2O2, including (a) an extensive crumb rubber literature review, (b) manual structural elucidation from mass spectra of the CEC, and (c) in silico fragmentation algorithms of the molecular ion peak at different ionization energies in software tools such as MetFrag and CSI:FingerID with matching against PubChem and ChemSpider databases. None of these approaches provided structural information.
The breakthrough for the structural elucidation came when the literature search included slight variations in the molecular formula based on the assumption that the causative agent could be an abiotic oxidative transformation product of a substance already used in tire rubber. Literature searches identified 6PPD with a molecular formula of C18H22N2 as a possible proto-toxicant. This hypothesis was tested when 6PPD was transformed using gas phase ozonation to a transformation product that was an exact match of the CEC isolated from the TWPL by UHPLC-HRMS and nuclear magnetic resonance (NMR) spectroscopy. The CEC was tentatively identified as 6PPD-q based on the parent compound and the structural information gleaned from the oxidation of 6PPD, which places the structural information about the CEC at Schymanski Confidence Level 3, or a tentative structural candidate identification (see Figure 3, Analytical Methods Fact Sheet). Schymanski Confidence Levels are defined solely by HRMS information and chromatography data (e.g., retention time at Schymanski Confidence Level 1) about the candidate molecule and do not account for structural confirmation using alternative instrumental techniques. The fact that this study confirmed the structure of the causative toxicant using NMR would place the confidence in its structure at a higher confidence level that is not mapped by the Schymanski Confidence Level Scale (see Figure 3, Analytical Methods Fact Sheet). Note that no analytical reference standards for 6PPD-q were commercially available by the end of the experimental study to help improve confidence in the structural information of the CEC to Schymanski Confidence Level 1 using UHPLC-HRMS or high-performance liquid chromatography (HPLC)-HRMS data.
3. Next Steps
The NTA case study described above used an EDA approach coupled with physical simplification processes for environmental mixture complexity reduction and UHPLC-HRMS, to determine the molecular formula of the toxicant responsible for URMS in coho salmon. Once the molecular formula was identified, the chemical structure was elucidated by testing an ozonation hypothesis using multiple instrumental analysis tools (HRMS and NMR).
The next challenge after determining the identity of a previously unknown CEC (i.e., molecular formula and chemical structure) is the development of a targeted quantitative analytical method that can determine concentrations of the toxicant in environmental media at trace levels. This challenge usually takes the following steps:
- Chemical synthesis and purification of an analytical reference standard (note that for 6PPD-q, the chemical synthesis could be from the proto-toxicant, 6PPD, which is widely available)
- Development of an analytical method for identification and quantitation of 6PPD-q within environmental media (note that HPLC-MS/MS is considered the “gold standard” for the most reliable and sensitive targeted analysis of semi- and nonvolatile organic compounds)
- Addressing any environmental media-specific issues, including stability and adsorption, that might require more specialized approaches such as derivatization or isotope dilution to account for potential matrix bias
4. Epilogue
EDA is only one NTA method. In the study reported here, EDA worked well as the CEC induced rapid and acute mortality in the test organisms at low concentrations. It should be noted, however, that most environmental toxicants demonstrate nonlethal chronic effects and, therefore, may not be amenable to such an approach. In such cases, other NTA methods, including chemistry driven approaches (e.g., search for particular chemical signatures) and statistically driven approaches (e.g., spatiotemporal trends) (Hollender et al. 2019), might be more suitable to determine CEC that induce chronic toxicity effects.
Fact Sheet References
ASTM International. 2020. “Standard Practice for Collection of Water Samples with High, Medium, or Low Suspended Solids for Identification and Quantification of Microplastic Particles and Fibers.” ASTM. August 14, 2020. https://www.astm.org/d8332-20.html.
ASTM International. 2023. “Standard Practice for Development and Use (Preparation) of Samples for Collaborative Testing of Methods for Analysis of Sediments.” 2023. https://www.astm.org/d3975-93r19.html.
Aven, Terje, and Frederic Bouder. 2020. “The COVID-19 Pandemic: How Can Risk Science Help?” Journal of Risk Research 23 (7–8): 849–54. https://doi.org/10.1080/13669877.2020.1756383.
Bahroun, Najat H. O., John D. Perry, Stephen P. Stanforth, and John R. Dean. 2018. “Use of Exogenous Volatile Organic Compounds to Detect Salmonella in Milk.” Analytica Chimica Acta 1028 (October): 121–30. https://doi.org/10.1016/j.aca.2018.03.065.
Bălan, Simona Andreea, Vivek Chander Mathrani, Dennis Fengmao Guo, and André Maurice Algazi. 2021. “Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program.” Environmental Health Perspectives 129 (2): 025001. https://doi.org/10.1289/EHP7431.
Batson, Joshua, Gytis Dudas, Eric Haas-Stapleton, Amy L. Kistler, Lucy M Li, Phoenix Logan, Kalani Ratnasiri, and Hanna Retallack. 2021. “Single Mosquito Metatranscriptomics Identifies Vectors, Emerging Pathogens and Reservoirs in One Assay.” Edited by Dominique Soldati-Favre. eLife 10 (April): e68353. https://doi.org/10.7554/eLife.68353.
Bhattacharjee, Sourav. 2016. “DLS and Zeta Potential – What They Are and What They Are Not?” Journal of Controlled Release 235 (August): 337–51. https://doi.org/10.1016/j.jconrel.2016.06.017.
Bostanci, Nagihan, Melissa Grant, Kai Bao, Angelika Silbereisen, Franziska Hetrodt, Daniel Manoil, and Georgios N. Belibasakis. 2021. “Metaproteome and Metabolome of Oral Microbial Communities.” Periodontology 2000 85 (1): 46–81. https://doi.org/10.1111/prd.12351.
Buck, Robert C., James Franklin, Urs Berger, Jason M. Conder, Ian T Cousins, Pim de Voogt, Allan Astrup Jensen, Kurunthachalam Kannan, Scott A. Mabury, and Stefan P.J. van Leeuwen. 2011. “Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins.” Integrated Environmental Assessment and Management 7 (4): 513–41. https://doi.org/10.1002/ieam.258.
Bustin, Stephen A., Vladimir Benes, Jeremy A. Garson, Jan Hellemans, Jim Huggett, Mikael Kubista, Reinhold Mueller, et al. 2009. “The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments.” Clinical Chemistry 55 (4): 611–22. https://doi.org/10.1373/clinchem.2008.112797.
CDC. 2019. “AMD: Uncovering Emerging Threats.” October 11, 2019. https://www.cdc.gov/amd/what-we-do/emerging-threats.html.
CDC. 2022. “Emerging Infectious Diseases Summaries.” September 19, 2022. https://www.cdc.gov/media/eid-summaries.htm.
CDC. 2023a. “MicrobeNet.” 2023. https://microbenet.cdc.gov/.
CDC. 2023b. “National Center for Emerging and Zoonotic Infectious Diseases (NCEZID).” February 17, 2023. https://www.cdc.gov/ncezid/index.html.
Charbonnet, Joseph A., Carrie A. McDonough, Feng Xiao, Trever Schwichtenberg, Dunping Cao, Sarit Kaserzon, Kevin V. Thomas, et al. 2022. “Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High-Resolution Mass Spectrometry.” Environmental Science & Technology Letters 9 (6): 473–81. https://doi.org/10.1021/acs.estlett.2c00206.
Cheng, Wei, and Richard G. Compton. 2014. “Electrochemical Detection of Nanoparticles by ‘Nano-Impact’ Methods.” TrAC Trends in Analytical Chemistry, New Nanobiosensing Techniques and Bioanalysis, 58 (June): 79–89. https://doi.org/10.1016/j.trac.2014.01.008.
Djoumbou-Feunang, Yannick, Jarlei Fiamoncini, Alberto Gil-de-la-Fuente, Russell Greiner, Claudine Manach, and David S. Wishart. 2019. “BioTransformer: A Comprehensive Computational Tool for Small Molecule Metabolism Prediction and Metabolite Identification.” Journal of Cheminformatics 11 (1): 2. https://doi.org/10.1186/s13321-018-0324-5.
DOC, NIST. 2023. “NIST Chemistry WebBook.” National Institute of Standards and Technology. 2023. https://doi.org/10.18434/T4D303.
DOL. 2023. “Sampling and Analytical Methods | Occupational Safety and Health Administration.” 2023. https://www.osha.gov/chemicaldata/sampling-analytical-methods.
Dong, Huiyu, Amy A. Cuthbertson, and Susan D. Richardson. 2020. “Effect-Directed Analysis (EDA): A Promising Tool for Nontarget Identification of Unknown Disinfection Byproducts in Drinking Water.” Environmental Science & Technology 54 (3): 1290–92. https://doi.org/10.1021/acs.est.0c00014.
FDA. 2012. “Bad Bug Book.” 2012. https://www.fda.gov/media/83271/download.
FDA. 2019. “Guidelines for the Validation of Microbiological Methods for the FDA Foods Program, 3rd Edition.” October 17, 2019. https://www.fda.gov/media/83812/download.
FDA. 2023. “Bacteriological Analytical Manual (BAM).” FDA, March. https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam.
Gilligan, Peter. 2021. “Metabolomics, the Newest ‘Omic’ in Diagnostic Microbiology.” ASM.Org. December 10, 2021. https://asm.org/Articles/2021/December/Metabolomics,-The-Newest-Omic-in-Diagnostic-Microb.
Grossegesse, Marica, Felix Hartkopf, Andreas Nitsche, Lars Schaade, Joerg Doellinger, and Thilo Muth. 2020. “Perspective on Proteomics for Virus Detection in Clinical Samples.” Journal of Proteome Research 19 (11): 4380–88. https://doi.org/10.1021/acs.jproteome.0c00674.
GWPP. 2015. “Global Water Pathogen Project.” 2015. https://www.waterpathogens.org/.
Heithmar, Edward M. 2011. “Screening Methods for Metal-Containing Nanoparticles in Water.” October 4, 2011. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=238306.
Hogan, Catherine A., Pranav Rajpurkar, Hari Sowrirajan, Nicholas A. Phillips, Anthony T. Le, Manhong Wu, Natasha Garamani, et al. 2021. “Nasopharyngeal Metabolomics and Machine Learning Approach for the Diagnosis of Influenza.” eBioMedicine 71 (September): 103546. https://doi.org/10.1016/j.ebiom.2021.103546.
Hollender, Juliane, Emma L. Schymanski, Heinz P. Singer, and P. Lee Ferguson. 2017. “Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go?” Environmental Science & Technology 51 (20): 11505–12. https://doi.org/10.1021/acs.est.7b02184.
Hou, Jun, Hanlin Ci, Peifang Wang, Chao Wang, Bowen Lv, Lingzhan Miao, and Guoxiang You. 2018. “Nanoparticle Tracking Analysis versus Dynamic Light Scattering: Case Study on the Effect of Ca2+ and Alginate on the Aggregation of Cerium Oxide Nanoparticles.” Journal of Hazardous Materials 360 (October): 319–28. https://doi.org/10.1016/j.jhazmat.2018.08.010.
Houtz, Erika F., Christopher P. Higgins, Jennifer A. Field, and David L. Sedlak. 2013. “Persistence of Perfluoroalkyl Acid Precursors in AFFF-Impacted Groundwater and Soil.” Environmental Science & Technology 47 (15): 8187–95. https://doi.org/10.1021/es4018877.
Houtz, Erika F., and David L. Sedlak. 2012. “Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff.” Environmental Science & Technology 46 (17): 9342–49. https://doi.org/10.1021/es302274g.
ISO. 2017. “ISO/IEC 17025:2017.” ISO. November 2017. https://www.iso.org/standard/66912.html.
ISO. 2023. “ISO – 13 – Environment. Health Protection. Safety.” 2023. https://www.iso.org/ics/13/x/.
ITRC. 2013a. “ITRC EMD-2 – 4 Quantitative Polymerase Chain Reaction.” April 2013. https://projects.itrcweb.org/emd-2/#4%20Quantitative%20Polymerase.htm.
ITRC. 2013b. “ITRC EMD-2 – Appendix D Microbiology FAQs.” April 2013. https://projects.itrcweb.org/emd-2/#Appendix%20D%20Microbiology%20FAQ.htm%3FTocPath%3D_____21.
ITRC. 2013c. “ITRC EMD-2 – Welcome.” April 2013. https://projects.itrcweb.org/emd-2/#Welcome.htm%3FTocPath%3D_____1.
ITRC. 2017. “Naming Conventions and Physical and Chemical Properties of Per- and Polyfluoroalkyl Substances (PFAS).” 2017. https://pfas-1.itrcweb.org/fact_sheets_page/PFAS_Fact_Sheet_Naming_Conventions_April2020.pdf.
ITRC. 2022. “11 Sampling and Analytical Methods – PFAS — Per- and Polyfluoroalkyl Substances.” 2022. https://pfas-1.itrcweb.org/11-sampling-and-analytical-methods/.
ITRC. 2023a. “ITRC PFAS, Chapter 11 Sampling and Analytical Methods, Section 11.2 Analytical Methods/Techniques.” 2023. 11 Sampling and Analytical Methods.
ITRC. 2023b. “Microplastics Team Materials.” 2023. https://mp-1.itrcweb.org.
Jadhav, Snehal R., Rohan M. Shah, Avinash V. Karpe, Paul D. Morrison, Konstantinos Kouremenos, David J. Beale, and Enzo A. Palombo. 2018. “Detection of Foodborne Pathogens Using Proteomics and Metabolomics-Based Approaches.” Frontiers in Microbiology 9. https://www.frontiersin.org/articles/10.3389/fmicb.2018.03132.
Kanehisa, Minoru, Miho Furumichi, Yoko Sato, Masayuki Kawashima, and Mari Ishiguro-Watanabe. 2023. “KEGG for Taxonomy-Based Analysis of Pathways and Genomes.” Nucleic Acids Research 51 (D1): D587–92. https://doi.org/10.1093/nar/gkac963.
Kärrman, Anna, Leo W. Y. Yeung, Kyra M. Spaan, Frank Thomas Lange, Minh Anh Nguyen, Merle Plassmann, Cynthia A. de Wit, Marco Scheurer, Raed Awad, and Jonathan P. Benskin. 2021. “Can Determination of Extractable Organofluorine (EOF) Be Standardized? First Interlaboratory Comparisons of EOF and Fluorine Mass Balance in Sludge and Water Matrices.” Environmental Science: Processes & Impacts 23 (10): 1458–65. https://doi.org/10.1039/D1EM00224D.
Langevin, D., E. Raspaud, S. Mariot, A. Knyazev, A. Stocco, A. Salonen, A. Luch, et al. 2018. “Towards Reproducible Measurement of Nanoparticle Size Using Dynamic Light Scattering: Important Controls and Considerations.” NanoImpact 10 (April): 161–67. https://doi.org/10.1016/j.impact.2018.04.002.
Lehrstuhl Bioinformatik Jena. 2023. “Lehrstuhl Für Bioinformatik an Der FSU Jena.” 2023. https://bio.informatik.uni-jena.de/.
Lenz, Robin, Kristina Enders, Colin A. Stedmon, David M. A. Mackenzie, and Torkel Gissel Nielsen. 2015. “A Critical Assessment of Visual Identification of Marine Microplastic Using Raman Spectroscopy for Analysis Improvement.” Marine Pollution Bulletin 100 (1): 82–91. https://doi.org/10.1016/j.marpolbul.2015.09.026.
Li, Na, Qingqing Cai, Qing Miao, Zeshi Song, Yuan Fang, and Bijie Hu. 2021. “High-Throughput Metagenomics for Identification of Pathogens in the Clinical Settings.” Small Methods 5 (1): 2000792. https://doi.org/10.1002/smtd.202000792.
Li, Shubo, Yufeng Tian, Pingyingzi Jiang, Ying Lin, Xiaoling Liu, and Hongshun Yang. 2021. “Recent Advances in the Application of Metabolomics for Food Safety Control and Food Quality Analyses.” Critical Reviews in Food Science and Nutrition 61 (9): 1448–69. https://doi.org/10.1080/10408398.2020.1761287.
Loman, Nicholas J., Chrystala Constantinidou, Martin Christner, Holger Rohde, Jacqueline Z.-M. Chan, Joshua Quick, Jacqueline C. Weir, et al. 2013. “A Culture-Independent Sequence-Based Metagenomics Approach to the Investigation of an Outbreak of Shiga-Toxigenic Escherichia Coli O104:H4.” JAMA 309 (14): 1502–10. https://doi.org/10.1001/jama.2013.3231.
MassBank-Consortium. 2021. “MassBank/MassBank-Data: Release Version 2021.12.” Zenodo. https://doi.org/10.5281/ZENODO.5775684.
McDonough, Carrie A., Jennifer L. Guelfo, and Christopher P. Higgins. 2019. “Measuring Total PFASs in Water: The Tradeoff between Selectivity and Inclusivity.” Current Opinion in Environmental Science & Health, Drinking water contaminants, 7 (February): 13–18. https://doi.org/10.1016/j.coesh.2018.08.005.
Miyake, Yuichi, Nobuyoshi Yamashita, Pawel Rostkowski, Man Ka So, Sachi Taniyasu, Paul K. S. Lam, and Kurunthachalam Kannan. 2007. “Determination of Trace Levels of Total Fluorine in Water Using Combustion Ion Chromatography for Fluorine: A Mass Balance Approach to Determine Individual Perfluorinated Chemicals in Water.” Journal of Chromatography A 1143 (1): 98–104. https://doi.org/10.1016/j.chroma.2006.12.071.
Mohammed Taha, Hiba, Reza Aalizadeh, Nikiforos Alygizakis, Jean-Philippe Antignac, Hans Peter H. Arp, Richard Bade, Nancy Baker, et al. 2022. “The NORMAN Suspect List Exchange (NORMAN-SLE): Facilitating European and Worldwide Collaboration on Suspect Screening in High Resolution Mass Spectrometry.” Environmental Sciences Europe 34 (1): 104. https://doi.org/10.1186/s12302-022-00680-6.
MoNA. 2023. “MassBank of North America.” 2023. https://massbank.us/.
Moody, C. A., W. C. Kwan, J. W. Martin, D. C. Muir, and S. A. Mabury. 2001. “Determination of Perfluorinated Surfactants in Surface Water Samples by Two Independent Analytical Techniques: Liquid Chromatography/Tandem Mass Spectrometry and 19F NMR.” Analytical Chemistry 73 (10): 2200–2206. https://doi.org/10.1021/ac0100648.
mzCloud. 2023. “Advanced Mass Spectral Database.” 2023. https://www.mzcloud.org/.
Neves, Marta M. P. S., Henri P. A. Nouws, Cristina Delerue-Matos, and Daniel Martín-Yerga. 2020. “Electrochemical Detection and Characterization of Nanoparticles: A Potential Tool for Environmental Purposes.” Current Opinion in Electrochemistry, Environmental Electrochemistry: Physical and Nano Electrochemistry, 22 (August): 58–64. https://doi.org/10.1016/j.coelec.2020.04.007.
NIOSH. 2003. “NIOSH Manual of Analytical Methods, Chapters, 4th Edition, NIOSH, CDC.” 2003. https://www.cdc.gov/niosh/docs/2003-154/chaps.html.
NIST. 2012. “Tandem Mass Spectral Library.” NIST, November. https://www.nist.gov/programs-projects/tandem-mass-spectral-library.
OECD. 2018. “Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs).” May 4, 2018. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en.
Peter, Katherine T., Allison L. Phillips, Ann M. Knolhoff, Piero R. Gardinali, Carlos A. Manzano, Kelsey E. Miller, Manuel Pristner, et al. 2021. “Nontargeted Analysis Study Reporting Tool: A Framework to Improve Research Transparency and Reproducibility.” Analytical Chemistry 93 (41): 13870–79. https://doi.org/10.1021/acs.analchem.1c02621.
Place, Benjamin. 2023. “Suspect List of Possible Per- and Polyfluoroalkyl Substances (PFAS).” Application/vnd.openxmlformats-officedocument.spreadsheetml.sheet,text/plain. National Institute of Standards and Technology. https://doi.org/10.18434/MDS2-2387.
PubChem. 2023. “PubChem.” National Library of Medicine. 2023. https://pubchem.ncbi.nlm.nih.gov/.
Rajagopala, Seesandra V., Nicole G. Bakhoum, Suman B. Pakala, Meghan H. Shilts, Christian Rosas-Salazar, Annie Mai, Helen H. Boone, et al. 2021. “Metatranscriptomics to Characterize Respiratory Virome, Microbiome, and Host Response Directly from Clinical Samples.” Cell Reports Methods 1 (6): 100091. https://doi.org/10.1016/j.crmeth.2021.100091.
Rajapaksha, P., A. Elbourne, S. Gangadoo, R. Brown, D. Cozzolino, and J. Chapman. 2019. “A Review of Methods for the Detection of Pathogenic Microorganisms.” Analyst 144 (2): 396–411. https://doi.org/10.1039/C8AN01488D.
Ritter, Evelyn E., Margaret E. Dickinson, John P. Harron, David M. Lunderberg, Paul A. DeYoung, Alix E. Robel, Jennifer A. Field, and Graham F. Peaslee. 2017. “PIGE as a Screening Tool for Per- and Polyfluorinated Substances in Papers and Textiles.” Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 407 (September): 47–54. https://doi.org/10.1016/j.nimb.2017.05.052.
Robel, Alix E., Kristin Marshall, Margaret Dickinson, David Lunderberg, Craig Butt, Graham Peaslee, Heather M. Stapleton, and Jennifer A. Field. 2017. “Closing the Mass Balance on Fluorine on Papers and Textiles.” Environmental Science & Technology 51 (16): 9022–32. https://doi.org/10.1021/acs.est.7b02080.
Robert Koch Institute. 2023. “EMERGE: Efficient Response to Highly Dangerous and Emerging Pathogens at EU Level.” 2023. https://www.emerge.rki.eu/Emerge/EN/Home/Homepage_node.html.
Rockey, Nicole, Heather N. Bischel, Tamar Kohn, Brian Pecson, and Krista R. Wigginton. 2019. “The Utility of Flow Cytometry for Potable Reuse.” Current Opinion in Biotechnology 57 (June): 42–49. https://doi.org/10.1016/j.copbio.2018.12.009.
Royal Society of Chemistry. 2023. “ChemSpider.” 2023. http://www.chemspider.com/.
Ruttkies, Christoph, Emma L. Schymanski, Sebastian Wolf, Juliane Hollender, and Steffen Neumann. 2016. “MetFrag Relaunched: Incorporating Strategies beyond in Silico Fragmentation.” Journal of Cheminformatics 8 (1): 3. https://doi.org/10.1186/s13321-016-0115-9.
Savidis, George, William M. McDougall, Paul Meraner, Jill M. Perreira, Jocelyn M. Portmann, Gaia Trincucci, Sinu P. John, et al. 2016. “Identification of Zika Virus and Dengue Virus Dependency Factors Using Functional Genomics.” Cell Reports 16 (1): 232–46. https://doi.org/10.1016/j.celrep.2016.06.028.
Schymanski, Emma L., and Evan E. Bolton. 2021. “FAIRifying the Exposome Journal: Templates for Chemical Structures and Transformations.” Exposome. December 24, 2021. https://watermark.silverchair.com/osab006.pdf?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAs0wggLJBgkqhkiG9w0BBwagggK6MIICtgIBADCCAq8GCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMkGLT_P1CSmOY4REJAgEQgIICgAFhb_KkukdPdnfg10h6wKPygTioqR2E6yY0IQINJzQPOGqTmzLTIp7ap4FMAr8PxgZNA7GG5egEdN40h24sh-78aYy_tQGIFsPwA9KSzPAK9X3oEuOFvIEZStPjK_0MkS1wQj-dHJmRulcZjjQZqa-NFDSwlgRpXW41rs5M5_B4zdttKZoO0aSutuk49kD_oXHZCfw9eiBXRpNqzVzvN1xyNztkGqSLNCiYoBIZl-DImA2wTx79KtucqDlPNhitR1L_Ejqvjpr1LphIvaBLtMhuLglOOparj1gNbXiBkgfLkir9jj76AOYmdrGIQbHeMUIlgLyPmkW2DeXoACkjas-NZqweD66ho5TuLZqOr3QSgHGQoll_qpck7KBBvRpIDsQlkNHE4eo_EExFmyDd8d-Q73XmSvUyZIqmCVLer94zRGjbGantwrUwR-JVSYrUJhWeL7jw–sgZF7LwV9rvpdz8HXZfpPUmHo2FwcMmF72fzIpnVXKQf3TSSvjuff2mAEITO0El77gmyMIUX1zhfFdvimCwJQ83I8igorScLCvlEPi_BMGqwCbC_EVoQaHak7Vb0rJ5iFv_mKMKEO-n3V-NgMfPrWkMSGVEKzVU7YhjRZDn8vY7ssC1Ou-oV9QH9JdyQF0oZ1CdS1NTLLgsYlDLTP4r8MJthcMmXSRKT3PhKgIPTkZpCX-mEg42IkG4zpDxTeqXNRCU0wkLq1eRCBk1N42Rn0CTR0fxaaJ_24yj43D4C3GrUftHN-AbgiRXGOF6o97Rbo8N-0Ffvq65jxpVJMQTpPPqY_wWQnBcSNecp0gi0vETyb7xAlLQkcKW-4TqK7g1aS90yRwJ3-IbVU.
Schymanski, Emma L., Junho Jeon, Rebekka Gulde, Kathrin Fenner, Matthias Ruff, Heinz P. Singer, and Juliane Hollender. 2014. “Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence.” Environmental Science & Technology 48 (4): 2097–98. https://doi.org/10.1021/es5002105.
Shimadzu. 2020. “Analytical and Measuring Instruments for Microplastics.” June 2020. https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/brochures/13211/c10ge083.pdf.
Stein, Stephen. 2012. “Mass Spectral Reference Libraries: An Ever-Expanding Resource for Chemical Identification.” Analytical Chemistry 84 (17): 7274–82. https://doi.org/10.1021/ac301205z.
Syu, Guan-Da, Jessica Dunn, and Heng Zhu. 2020. “Developments and Applications of Functional Protein Microarrays.” Molecular & Cellular Proteomics 19 (6): 916–27. https://doi.org/10.1074/mcp.R120.001936.
Tian, Zhenyu, Haoqi Zhao, Katherine T. Peter, Melissa Gonzalez, Jill Wetzel, Christopher Wu, Ximin Hu, et al. 2020. “A Ubiquitous Tire Rubber–Derived Chemical Induces Acute Mortality in Coho Salmon.” Science 371 (6525): 185–89. https://doi.org/10.1126/science.abd6951.
USDA. 2020. “Illnesses and Pathogens | Food Safety and Inspection Service.” November 16, 2020. http://www.fsis.usda.gov/food-safety/foodborne-illness-and-disease/illnesses-and-pathogens.
USEPA. 2004. “Quality Assurance/Quality Control Guidance for Laboratories Performing PCR Analyses on Environmental Samples.” October 2004. https://projects.itrcweb.org/emd-2/Content/Resources/EPA-815-B-04-001.pdf.
USEPA. 2012. “SW-846 Method Style Guide.” June 25, 2012. https://www.epa.gov/sites/default/files/2015-10/documents/style_gd_062512.pdf.
USEPA. 2016. “Microbological Methods Validation Policy 2009.” 2016. https://www.epa.gov/sites/default/files/2017-07/documents/final_revision_microbiological_validity_policy_122116.pdf.
USEPA, OCSPP. 2014. “TSCA Chemical Substance Inventory.” Collections and Lists. August 15, 2014. https://www.epa.gov/tsca-inventory.
USEPA, OCSPP. 2023. “Chemicals under the Toxic Substances Control Act (TSCA).” Collections and Lists. August 16, 2023. https://www.epa.gov/chemicals-under-tsca.
USEPA, ORD. 2022. “SAM Chemical Methods Query.” Collections and Lists. July 18, 2022. https://www.epa.gov/esam/sam-chemical-methods-query.
USEPA, ORD. 2023. “CompTox Chemicals Dashboard.” Data and Tools. February 23, 2023. https://www.epa.gov/chemical-research/comptox-chemicals-dashboard.
USEPA, OW. 2020. “Frequent Questions about PFAS Methods for NPDES Permits.” Reports and Assessments. United States. November 29, 2020. https://www.epa.gov/cwa-methods/frequent-questions-about-pfas-methods-npdes-permits.
USEPA, OW. 2022. “Draft Method 1621: Screening Method for the Determination of Adsorbable Organic Fluorine (AOF) in Aqueous Matrices by Combustion Ion Chromatography.” April 2022. https://www.epa.gov/system/files/documents/2022-04/draft-method-1621-for-screening-aof-in-aqueous-matrices-by-cic_0.pdf.
USGS, NEMI. 2023. “National Environmental Methods Index.” 2023. https://www.nemi.gov/home/.
Wagner, Andrea, Brigitte Raue, Heinz-Jürgen Brauch, Eckhard Worch, and Frank T. Lange. 2013. “Determination of Adsorbable Organic Fluorine from Aqueous Environmental Samples by Adsorption to Polystyrene-Divinylbenzene Based Activated Carbon and Combustion Ion Chromatography.” Journal of Chromatography A 1295 (June): 82–89. https://doi.org/10.1016/j.chroma.2013.04.051.
Willach, Sarah, Heinz-Jürgen Brauch, and Frank T. Lange. 2016. “Contribution of Selected Perfluoroalkyl and Polyfluoroalkyl Substances to the Adsorbable Organically Bound Fluorine in German Rivers and in a Highly Contaminated Groundwater.” Chemosphere 145 (February): 342–50. https://doi.org/10.1016/j.chemosphere.2015.11.113.
Wong, Charles S. 2021. “Standard Operating Procedures for Extraction and Measurement by Raman Spectroscopy of Microplastic Particles in Drinking Water.” Ca.Gov. September 24, 2021. https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/documents/microplastics/mcrplstcs_raman.pdf.
Yegin, Betül Arıca, and Alf Lamprecht. 2006. “Lipid Nanocapsule Size Analysis by Hydrodynamic Chromatography and Photon Correlation Spectroscopy.” International Journal of Pharmaceutics 320 (1): 165–70. https://doi.org/10.1016/j.ijpharm.2006.04.014.
Zhang, Ming, Junhan Yang, Zhongxia Cai, Yudong Feng, Yafeng Wang, Daoyong Zhang, and Xiangliang Pan. 2019. “Detection of Engineered Nanoparticles in Aquatic Environments: Current Status and Challenges in Enrichment, Separation, and Analysis.” Environmental Science: Nano 6 (3): 709–35. https://doi.org/10.1039/C8EN01086B.
Case Study References
Chow, Michelle I., Jessica I. Lundin, Chelsea J. Mitchell, Jay W. Davis, Graham Young, Nathaniel L. Scholz, and Jenifer K. McIntyre. 2019. “An Urban Stormwater Runoff Mortality Syndrome in Juvenile Coho Salmon.” Aquatic Toxicology (Amsterdam, Netherlands) 214 (September): 105231. https://doi.org/10.1016/j.aquatox.2019.105231.
Dong, Huiyu, Amy A. Cuthbertson, and Susan D. Richardson. 2020. “Effect-Directed Analysis (EDA): A Promising Tool for Nontarget Identification of Unknown Disinfection Byproducts in Drinking Water.” Environmental Science & Technology 54 (3): 1290–92. https://doi.org/10.1021/acs.est.0c00014.
Hollender, Juliane, Bert van Bavel, Valeria Dulio, Eivind Farmen, Klaus Furtmann, Jan Koschorreck, Uwe Kunkel, et al. 2019. “High Resolution Mass Spectrometry-Based Non-Target Screening Can Support Regulatory Environmental Monitoring and Chemicals Management.” Environmental Sciences Europe 31 (1): 42. https://doi.org/10.1186/s12302-019-0225-x.
Peter, Katherine T., Zhenyu Tian, Christopher Wu, Peter Lin, Sarah White, Bowen Du, Jenifer K. McIntyre, Nathaniel L. Scholz, and Edward P. Kolodziej. 2018. “Using High-Resolution Mass Spectrometry to Identify Organic Contaminants Linked to Urban Stormwater Mortality Syndrome in Coho Salmon.” Environmental Science & Technology 52 (18): 10317–27. https://doi.org/10.1021/acs.est.8b03287.
Tian, Zhenyu, Haoqi Zhao, Katherine T. Peter, Melissa Gonzalez, Jill Wetzel, Christopher Wu, Ximin Hu, et al. 2021. “A Ubiquitous Tire Rubber-Derived Chemical Induces Acute Mortality in Coho Salmon.” Science (New York, N.Y.) 371 (6525): 185–89. https://doi.org/10.1126/science.abd6951.