1. INTRODUCTION
This case study describes an effect-directed nontargeted analysis (NTA) experimental study (Tian et al. 2021) to identify the contaminant of emerging concern (CEC) responsible for acute mortality in coho salmon (Oncorhynchus kisutch) observed during runoff events in the Pacific Northwest (a.k.a. “urban runoff mortality syndrome” [URMS]). URMS occurs among adult coho salmon populations annually when they return to spawn in freshwater, especially to waters located in urbanized watersheds. Effect-directed analysis (EDA) is a strategy for environmental profiling of samples that combines sample fractionation schemes, toxic effect assays, and chemical analysis to identify toxic pollutants (Dong, Cuthbertson, and Richardson 2020). More recently, EDA has been paired with NTA using high-resolution mass spectrometry (HRMS) for chemical analysis to identify CEC in complex environmental samples (Dong, Cuthbertson, and Richardson 2020; Hollender et al. 2019). The case study begins with tire leachate (i.e., tread wear particle leachate [TWPL]) in the “known-unknowns” quadrant of the Rumsfeld matrix (see Figure 1, Analytical Methods Fact Sheet) after targeted analysis methods had been exhausted and after a chemical compositional similarity had been established among TWPL, roadway runoff, and URMS–associated waters (Peter et al. 2018).
2. EXPERIMENTAL METHODOLOGY AND RESULTS
The experimental design of the study relied upon three key tools for identifying the CEC:
- A fractionation scheme to simplify the complex TWPL mixture
- Acute exposure toxicity assays to screen TWPL fractions for their ability to induce mortality in juvenile coho salmon
- Ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS) for structural determination (i.e., by molecular formula determination using accurate mass—see Figure 1)
Acute toxicity assays were performed on juvenile coho salmon as a proxy for adult coho (Chow et al. 2019). The CEC was eventually determined to be a quinone oxidation byproduct of the tire antioxidant N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine (6PPD), or 6PPD-quinone (6PPD-q). Note that only a cursory description of follow-up work beyond the molecular formula determination (i.e., to elucidate the chemical structure of the CEC) is provided within this case study writeup. The subsections that follow describe various stages of the study, which are also graphically summarized in Figure 2.
Figure 1. Accurate mass from HRMS for determining molecular formula. HRMS yields ion mass at an accuracy of three to seven decimal places (i.e., 0.001 Dalton [Da] [or amu] or less), thereby deciphering its unique molecular formula. A conventional unit‐resolution quadrupole mass spectrometer cannot distinguish the identity of a hypothetical 60 amu or Da (60 m/z) nominal mass molecular ion peak among four different compounds (i.e., acetic acid, urea, propan‐1‐ol, and ethylenediamine); however, a molecular ion peak of 60.0687 amu obtained using HRMS (e.g., by using a quadrupole‐time‐of‐flight mass spectrometer) would clearly indicate the compound ethylenediamine.
Source: ITRC CEC Team
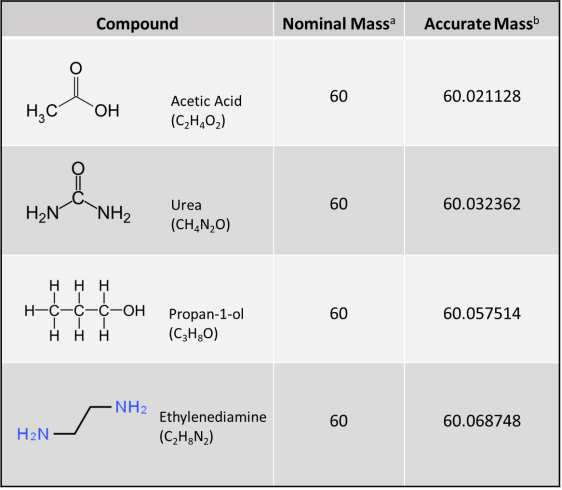
a. Nominal mass is at unit resolution and is expressed as an integer. For monoisotopic ions (i.e., ions made up of only the most abundant isotope of each of its constituent elements), nominal masses of elements in atomic mass units (amu) or Daltons are C=12, H=1, N=14, and O=16.
b. Accurate mass is obtained from a high-resolution instrument that gives a molecular mass at an accuracy of three to seven decimal places. For monoisotopic ions, accurate masses of elements in amu or Daltons are C=12.000000, H=1.007825, N=14.003074, and O=15.994914.
Figure 2. Overall schema for 6PPD‐q effect‐directed analysis (EDA) study
Source: Tian and colleagues (2021).

2.1 Stage I. Preparation and Testing of TWPL
An aqueous TWPL was prepared using tread particles from nine different used and new tires. The TWPL was found to be lethal (98.5% mortality in <6 hours) to juvenile coho salmon in a 24-hour acute toxicity test. A negative control demonstrated no mortality while toxicity remained unabated in a heated control, indicating stability during handling, and in a particle-free control, indicating that the CEC was dissolved and not particle-associated. Toxicity also remained unchanged after the leachate was treated with ion exchange resins (cation and anion) and ethylenediaminetetraacetic acid (EDTA) indicating that the CEC was not ionized and was not a metal, respectively. UHPLC-HRMS of the TWPL in positive and negative electrospray ionization modes (i.e., ESI+ and ESI–, respectively) produced more than 2,000 combined chemical features with unique mass to charge ratio (m/z) and retention time, indicating the complexity of the TWPL mixture. This necessitated cleanup of the mixture in Stage 2 to generate a simplified yet toxic mixture more amenable to individual compound identification.
2.2 Stage II. Suspect Screening and Individual Compound Toxicity Testing
In Stage II, the TWPL underwent sequential cleanup using cation exchange, silica gel, and reversed-phase C-18 separation. Fractions were collected and were subjected to acute toxicity testing. Only one fraction (Fraction 6 or F6) produced acute mortality in coho salmon, indicating that it contained the suspected CEC. The fraction was analyzed using UHPLC-HRMS, which resulted in approximately 225 and 70 chemical features in ESI+ and ESI– ionization modes, respectively. This constituted a significant reduction in sample complexity because only 10% of the features found in the initial TWPL mixture remained in the active fraction. A suspect screening list was compiled using an in-house database of tire rubber compounds (n=258) and a larger database from the NORMAN (network of reference laboratories, research centers and related organizations for monitoring of emerging environmental substances) Suspect List Exchange Database of approximately 30,000 environmental pollutants. These lists were integrated into the data-dependent acquisition to prioritize tandem mass spectral (MS/MS) collection for suspected compounds. Eleven compounds, including plasticizers, antioxidants, emulsifiers, and their transformation products, were identified in the active fraction but none induced mortality in the test organisms. The negative results from the suspect screening stage led the authors to conclude that the CEC responsible for URMS was previously unreported in scientific literature and required an NTA approach for identification.
2.3 Stage III. NTA: Mixture Complexity and Chemical Feature Reduction
A parallel preparative-level chromatography approach was employed to further reduce the mixture complexity and limit the number of chemical features. Columns with different stationary phases (C-18, pentafluorophenyl [PFP], and phenyl), together with fraction collection, were used to change the elution order of the CEC, as well as the mixture composition of each fraction. Only fractions C-18-F6, PFP-F6, and Phenyl-F4 induced acute mortality in test organisms. These toxic fractions were independently analyzed using UHPLC-HRMS and resulted in the ESI+ chemical features of 251 for C-18-F6, 204 for PFP-F6, and 237 for Phenyl-F4. Similarly, ESI– chemical features of 77 for C-18-F6, 60 for PFP-F6, and 75 for Phenyl-F4 were obtained. Common chemical features present in all three lethal fractions were identified, resulting in only four ESI+ and three ESI– chemical features.
Peaks for these common chemical features were prioritized by abundance, resulting in the identification of a dominant chemical feature (i.e., 10-fold higher intensity in both ESI+ and ESI– ) that that had a measured mass of 298.1681 atomic mass units (amu) (or ESI+ [M+H] protonated peaks of 299.1752–299.1777 amu). After subtracting the proton mass, this molecular ion peak corresponded to the monoisotopic ion (i.e., an ion composed of only the most abundant isotope of each element) with a molecular formula of C18H22N2O2. The structural information gleaned about the CEC at this stage would place it at Level 5 on the Schymanski Confidence Level Scale (see Figure 3, Analytical Methods Fact Sheet), where the exact mass of interest had been identified, but remained a high-priority CEC based on the toxicity results.
2.4 Stage IV. CEC Purification
The preparative chromatography setup in Stage III was converted from parallel to serial (i.e., C-18 PFP Phenyl) to recover a sufficient amount of material to facilitate chemical structure studies in Stage V. Solvent removal via centrifugal evaporation and toxicity confirmation between separations yielded a pink-magenta precipitate that was dominated by the C18H22N2O2 molecular ion peak via UHPLC-HRMS (chemical features: ESI+ = 4 and ESI‐ = 3). The material caused 100% mortality in juvenile coho salmon upon 4 hours of exposure.
2.5 Stage V. Structural Confirmation and Additional Testing
Various tools were employed to elucidate the structure of the CEC with a molecular formula of C18H22N2O2, including (a) an extensive crumb rubber literature review, (b) manual structural elucidation from mass spectra of the CEC, and (c) in silico fragmentation algorithms of the molecular ion peak at different ionization energies in software tools such as MetFrag and CSI:FingerID with matching against PubChem and ChemSpider databases. None of these approaches provided structural information.
The breakthrough for the structural elucidation came when the literature search included slight variations in the molecular formula based on the assumption that the causative agent could be an abiotic oxidative transformation product of a substance already used in tire rubber. Literature searches identified 6PPD with a molecular formula of C18H22N2 as a possible proto-toxicant. This hypothesis was tested when 6PPD was transformed using gas phase ozonation to a transformation product that was an exact match of the CEC isolated from the TWPL by UHPLC-HRMS and nuclear magnetic resonance (NMR) spectroscopy. The CEC was tentatively identified as 6PPD-q based on the parent compound and the structural information gleaned from the oxidation of 6PPD, which places the structural information about the CEC at Schymanski Confidence Level 3, or a tentative structural candidate identification (see Figure 3, Analytical Methods Fact Sheet). Schymanski Confidence Levels are defined solely by HRMS information and chromatography data (e.g., retention time at Schymanski Confidence Level 1) about the candidate molecule and do not account for structural confirmation using alternative instrumental techniques. The fact that this study confirmed the structure of the causative toxicant using NMR would place the confidence in its structure at a higher confidence level that is not mapped by the Schymanski Confidence Level Scale (see Figure 3, Analytical Methods Fact Sheet). Note that no analytical reference standards for 6PPD-q were commercially available by the end of the experimental study to help improve confidence in the structural information of the CEC to Schymanski Confidence Level 1 using UHPLC-HRMS or high-performance liquid chromatography (HPLC)-HRMS data.
3. NEXT STEPS
The NTA case study described above used an EDA approach coupled with physical simplification processes for environmental mixture complexity reduction and UHPLC-HRMS, to determine the molecular formula of the toxicant responsible for URMS in coho salmon. Once the molecular formula was identified, the chemical structure was elucidated by testing an ozonation hypothesis using multiple instrumental analysis tools (HRMS and NMR).
The next challenge after determining the identity of a previously unknown CEC (i.e., molecular formula and chemical structure) is the development of a targeted quantitative analytical method that can determine concentrations of the toxicant in environmental media at trace levels. This challenge usually takes the following steps:
- Chemical synthesis and purification of an analytical reference standard (note that for 6PPD-q, the chemical synthesis could be from the proto-toxicant, 6PPD, which is widely available)
- Development of an analytical method for identification and quantitation of 6PPD-q within environmental media (note that HPLC-MS/MS is considered the “gold standard” for the most reliable and sensitive targeted analysis of semi- and nonvolatile organic compounds)
- Addressing any environmental media-specific issues, including stability and adsorption, that might require more specialized approaches such as derivatization or isotope dilution to account for potential matrix bias
4. EPILOGUE
EDA is only one NTA method. In the study reported here, EDA worked well as the CEC induced rapid and acute mortality in the test organisms at low concentrations. It should be noted, however, that most environmental toxicants demonstrate nonlethal chronic effects and, therefore, may not be amenable to such an approach. In such cases, other NTA methods, including chemistry driven approaches (e.g., search for particular chemical signatures) and statistically driven approaches (e.g., spatiotemporal trends) (Hollender et al. 2019), might be more suitable to determine CEC that induce chronic toxicity effects.
References
Chow, Michelle I., Jessica I. Lundin, Chelsea J. Mitchell, Jay W. Davis, Graham Young, Nathaniel L. Scholz, and Jenifer K. McIntyre. 2019. “An Urban Stormwater Runoff Mortality Syndrome in Juvenile Coho Salmon.” Aquatic Toxicology (Amsterdam, Netherlands) 214 (September): 105231. https://doi.org/10.1016/j.aquatox.2019.105231.
Dong, Huiyu, Amy A. Cuthbertson, and Susan D. Richardson. 2020. “Effect-Directed Analysis (EDA): A Promising Tool for Nontarget Identification of Unknown Disinfection Byproducts in Drinking Water.” Environmental Science & Technology 54 (3): 1290–92. https://doi.org/10.1021/acs.est.0c00014.
Hollender, Juliane, Bert van Bavel, Valeria Dulio, Eivind Farmen, Klaus Furtmann, Jan Koschorreck, Uwe Kunkel, et al. 2019. “High Resolution Mass Spectrometry-Based Non-Target Screening Can Support Regulatory Environmental Monitoring and Chemicals Management.” Environmental Sciences Europe 31 (1): 42. https://doi.org/10.1186/s12302-019-0225-x.
Peter, Katherine T., Zhenyu Tian, Christopher Wu, Peter Lin, Sarah White, Bowen Du, Jenifer K. McIntyre, Nathaniel L. Scholz, and Edward P. Kolodziej. 2018. “Using High-Resolution Mass Spectrometry to Identify Organic Contaminants Linked to Urban Stormwater Mortality Syndrome in Coho Salmon.” Environmental Science & Technology 52 (18): 10317–27. https://doi.org/10.1021/acs.est.8b03287.
Tian, Zhenyu, Haoqi Zhao, Katherine T. Peter, Melissa Gonzalez, Jill Wetzel, Christopher Wu, Ximin Hu, et al. 2021. “A Ubiquitous Tire Rubber-Derived Chemical Induces Acute Mortality in Coho Salmon.” Science (New York, N.Y.) 371 (6525): 185–89. https://doi.org/10.1126/science.abd6951.